Answers for Study Guide for Chemistry Test on Acids, Bases, and
advertisement

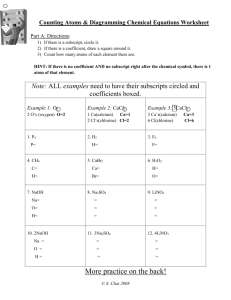
Answers for Study Guide for Chemistry Test (Acids-Bases; Reactants/Products; &Conservation of Matter) 1. What are the 5 signs that a chemical reaction has occurred? a. change of color b. a precipitate may form c. a gas may form d. temperature may change e. a new substance is made with different properties from the reactant 2. In a chemical equation, where are the reactants written? The reactants are always to the left of the arrow. They are written first. 3. In a chemical equation, where are the products written? The products are always to the right of the arrow. They are written last. 4. What does the arrow mean in a chemical equation? The arrow means produce. 5. What do reactants always form in a chemical reaction? They form a product. 6. What is the total number of atoms of Oxygen in this product: C6H12O6+ 6O2 There are 6 + 12 atoms of Oxygen in this product which means there are 18 atoms of Oxygen. 7. What does the Law of Conservation of Matter state? This law states that matter cannot be created nor destroyed in a chemical reaction. It only changes forms so the mass of the reactants must equal the mass of the product. 8. What must always be true of a chemical equation if it supports the Law of Conservation of Matter? The equation must be balanced. That means the mass of the reactants must equal the mass of the product. 9. What can you add to chemical equations to balance them? You may only add coefficients to balance chemical equations. 10. Prove whether or not these chemical equations are balanced: SO2 + O2 + 2H2O 4H2SO4 S=1 S=4 **NOT BALANCED** O =2+2+2 O = 16 **NOT BALANCED** H=4 H=8 **NOT BALANCED** 2Fe2 O3 + 3C 4Fe + 3 CO2 Fe = 4 Fe = 4 O=6 O=6 C=3 C=3 **BALANCED** **BALANCED** **BALANCED** 11. When you add two chemicals together and a new solid is formed, what is the solid that falls to the bottom called? The solid is called a precipitate . 12. What are the properties of an acid? a. Taste sour and are watery b. Corrosive to metals c. Litmus paper will be red d. Neutralizes with a base 13. What are the properties of a base (alkali)? a. Taste bitter and are slippery b. Corrosive to metals c. Litmus paper will be blue d. Neutralizes with an acid 14. What is an indicator? An indicator is a substance that will turn different colors in an acid or a base. 15. What numbers on the pH scale indicate that the solution is an acid? Acids range from 0 to 6.9 on the pH scale. 16. What number on the pH scale indicates that the solution is a base/alkali? Bases range from 7.9 to 14 on the pH scale. 17. What does the number 7 on the pH scale mean? What solution will be 7? The number 7 means that the solution is neutral. Water is a neutral solution. 18. When red litmus turns blue, what does that tell you about the solution? When the red litmus turns blue, that means the solution is a base. 19. When blue litmus turns red, what does that tell you about the solution? When blue litmus turns red, that means the solution is an acid. 20. When using litmus paper, blue means base or alkali and red means acid. 21. When you are using phenolphthalein, and it turns hot pink or magenta, what is that solution? When the phenolphthalein turns hot pink or magenta that means that the solution is a base. 22. When the phenolphthalein stays clear in a solution, what is that solution? When the phenolphthalein stays clear, that means that the solution is an acid or neutral. 23. What are some common solutions that are bases? Common base solutions are soaps, Drano, Windex, baking soda, ammonia, cleaning products, and quick lime from the ground. 24. What are some common solutions that are acids? Common acid solutions are lemon juice, vinegar, milk, hydrochloric acid, and Coke 25. When Calcium Chloride was mixed with water, it heated up very quickly. What type of change would this be? Exothermic chemical change 26. When Baking Soda is mixed with vinegar, it produces lots of gas bubbles and cools down quickly. What type of chemical reaction is this? Endothermic chemical change