Ch 8 Lecture Notes
advertisement
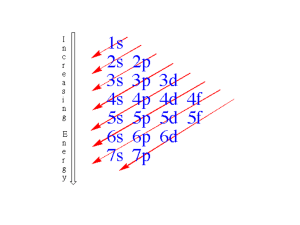
Table of Contents Chapter 8 – Electron Configurations and Chemical Periodic Trends I. ELECTRON SPIN ................................................................................................................................................ 2 THE 4TH QUANTUM NUMBER (MS)................................................................................................................................. 2 PARAMAGNETIC / DIAMAGNETIC BEHAVIOR ............................................................................................................... 2 II. ELECTRON CONFIGURATIONS AND ENERGY DIAGRAMS ............................................................. 3 ENERGY LEVEL DIAGRAMS ......................................................................................................................................... 3 Energy Levels ......................................................................................................................................................... 3 3 PRINCIPLES FOR ORDER OF FILLING ENERGY LEVELS .............................................................................................. 4 ATOMIC ELECTRON CONFIGURATIONS ........................................................................................................................ 4 ELECTRON CONFIGURATIONS – EXCEPTIONS TO THE RULES ....................................................................................... 5 ION ELECTRON CONFIGURATIONS ............................................................................................................................... 6 Determining Ion Electron Configurations .............................................................................................................. 6 Predicting Oxidation States .................................................................................................................................... 6 III. CHEMICAL PERIODIC TRENDS ................................................................................................................ 7 EFFECTIVE NUCLEAR CHARGE - ZEFF OR Z* ................................................................................................................. 8 Effective vs Inefective screening ............................................................................................................................. 9 ATOMIC SIZE ............................................................................................................................................................... 9 Trends in Atomic Size ........................................................................................................................................... 10 IONIC RADII ............................................................................................................................................................... 10 Cations .................................................................................................................................................................. 10 Anions ................................................................................................................................................................... 11 Isoelectronic Species ............................................................................................................................................ 11 IONIZATION ENERGY ................................................................................................................................................. 12 1st Ionization Energy Ei1 ...................................................................................................................................... 12 2nd Ionization Energy Ei2 ..................................................................................................................................... 12 Trends in Ionization Energy ................................................................................................................................. 13 Anomolies in Ionization Energy ............................................................................................................................ 13 ELECTRON AFFINITY ................................................................................................................................................. 14 Page 8-1 Chapter 8 – Electron Configurations and Chemical Periodic Trends I. Electron Spin The 4th Quantum number (ms) - Did not come directly from the wave equation. - It determines the - It can have values of on the electron. or . Pictue from McMurry Fay - - Spinning electrons (charged species) generate a . - - When placed in a magnetic field they will align themselves either the field. (They will be either or by or to the field.) - When the first e- is assigned to an orbital, it can have either value of . - - When a second electron is assigned to the same orbital, it will have the spin. Paramagnetic / Diamagnetic Behavior When placed into a magnetic field: - - Diamagnetic substances are all the field and have . - - Paramagnetic substances are at least 1 the field and have . - - Ferromagnetic substances are all unpaired electrons the field and have with the field. (e.g. Fe; These are permanent magnets.) Page 8-2 II. Electron Configurations and Energy Diagrams Energy Level Diagrams - Energy levels increase in the same order as the order of the . - For all atoms except hydrogen, the energy associated with an orbital is dependent on the values of (or the sublevel). , Pictue from McMurry Fay Energy Levels Since each set of quantum numbers is unique, you would expect that the P.E. of each orbital would also be unique, however: - The P.E. for some orbitals are the same and result in ____________ orbitals. - For the H atom, all orbitals with the same value of are degenerate. - For other atoms, all orbitals with the same values of are degenerate. Sample Full Energy Level Diagram ____ ____ 5p ____ Pictue from McMurry Fay ____ ____ ____ 4d ____ ____ ____ ____ ____ 3d ____ ____ ____ 5s ____ ____ 4p ____ ____ 4s ____ ____ 3p ____ ____ 2p ____ Energy spacing is not equivalent, see above diagram. ____ 3s ____ ____ 2s ____ 1s Page 8-3 An orbital box diagram simply lists the above energy levels sequentially: ____ ____ ____ ____ ____ ____ ____ ____ ____ ____ etc. 1s 2s 2p 3s 3p 4s 3 Principles for Order of Filling Energy Levels Aufbau Principle Hund’s Rule Always fill the energy levels first. If you have orbitals of the same energy ( you fill 1 in each orbital before you double up. All unpaired e- all have the spin. Pauli Exclusion Principle No two electrons can have the same ), . Atomic Electron Configurations A summary of the orbital location of each electron is written as, for example: Mg 1s2 2s2 2p6 3s2 etc. The primary QN (n) or level The sublevel or orbital type The number of electrons in the sublevel Class Practice Determine the electron configuration and orbital box diagram for: H He Li C V OR OR 1 s1 1s2 1s2 2s1 1s2 2s2 2p2 1s2 2s2 2p6 3s2 3p6 4s2 3d3 [Ar] 4s2 3d3 [Ar] 3d3 4s2 This can be called often re-written with the electrons last notation or a Have class try: W ________________________________________ Page 8-4 and notation. An alternate trick that can be used to determine the order or sublevel filling is: http://www.chemistry.ohio-state.edu/~grandinetti/teaching/Chem121/lectures/ Electron Configurations – Exceptions to the Rules - There are 19 out of 109 elements whose actual electronic configuration does not match what would be predicted by the rules that we have learned. - This occurs because orbital energies start to get closer and closer together as we get farther from the nucleus, and other contributions to the of the atom can change the filling order. - and subshells are usually more stable (lower energy). - You are responsible to know 5 of the 19 exceptions, which fall into 1 of 2 situations. Cr and Mo Cr [Ar] 4s2 3d4 predicted [Ar] 4s 3d ___ 4s 3d . becomes [Ar] When you move one electron up from the 4s to the 3d sublevel, they both become half full . Cr [Ar] 4s1 3d5 actual Mo [Kr] 5s2 4d4 predicted __________________ Page 8-5 actual Cu, Ag, an Au Cu [Ar] 4s2 3d9 predicted [Ar] 4s 3d . 4s 3d When you move one electron up from the 4s to the 3d sublevel, you get one full and one half full sublevel. becomes [Ar] Cu [Ar] 4s1 3d10 actual Ag [Kr] 5s2 4d9 predicted __________________ actual Au [Xe] 6s2 5d9 predicted __________________ actual Ion Electron Configurations Determining Ion Electron Configurations Calcium ion Ca [Ar] 4s2 Ca2+ [Ar] + + 2e2e- nitride ion N + 3e2 3 [He] 2s 2p + 3e- N3[He] 2s2 2p6 or [Ne] species (having the same # of electron configuration ) have the same example: N3-, O2-, F-, Ne, Na+, Mg2+, Al3+ are isoelectronic They have the same # of electrons, but have different numbers of protons. All have the configuration: . Predicting Oxidation States The most common oxidation states of metals is based on a gain or loss of electrons that will leave a electron configuration. Iron forms two common oxidation states Fe2+ and Fe3+. Page 8-6 Why? Basic Rules: Transition Metals will loose their outer inner electrons. electrons before their e.g., Ag Ag [Kr] 5s1 4d10 Ag [Kr] 4d10 5s1 _____________________ e.g., Iron Fe [Ar] 4s2 3d6 Fe [Ar] 3d6 4s2 ____________________ The d subshell would be more stable if half filled so Fe continues on: Fe2+ [Ar] 3d6 _____________________ Main Group Metals loose their electrons before their electrons. e.g., Lead Pb [Xe] 6s2 4f14 5d10 6p2 Pb [Xe] 4f14 5d10 6s2 6p2 ________________________ The configuration would also be stable without the s subshell so Pb continues on: Pb2+ [Xe] 4f14 5d10 6s2 _________________________ III. Chemical Periodic Trends - Many chemical and physical properties of the elements exhibit regular trends. - Demitri Mendeleev used thes similarities to help arrange his periodic table. Groups or families have similar behavior. - We will try to use what we now know about electronic structure to explain this. All Group 1 have ______ config “ 2 have ______ “ “ 3 have ______ “ similar configurations lead to “ 17 have ______ “ “ 18 have ______ “ Page 8-7 similar properties. Effective Nuclear Charge - Zeff or Z* - The predicted nuclear charge, , is based on the number of ___________ in the nucleus. Each positive charge adds to Z. - Inner electrons shield the positive nucleus from the outer electrons so that they feel less of an attractive force from the positive nucleus. - The actual positive charge that an electron experiences is the , Zeff or Z* The effective charge is reduced from the full charge due to the shielding of the nuclear charge by other electron in the atom. This picture is taken from http://www.chemistry.ohiostate.edu/~grandinetti/teachin g/Chem121/lectures/ The effective nuclear charge equates the number of protons in the nucleus, Z, minus the average number of electrons, S, between the nucleus and the electron of interest. picture was taken from: http://www.chemistry.ohiostate.edu/~grandinetti/ teaching/Chem121/lectures/ This Page 8-8 Effective vs Inefective screening - Inner shell electrons provide (but not complete) screening, because they lie mostly between the outer electrons and the nucleus. - Same shell electrons provide (negligible) screening, because they are at a similar distance from the nucleus. - Z* is somewhere between the true charge from the nucleus and the chage if screening were complete. Example: Lithium has 3 protons, so the maximum pull from the nucleus would be Lithium also has 3 electrons: inner 1s electrons and . valence (outer) 2s e-. If the inner electrons provided no screening, Z would be . If each of the inner e- could completely screen 1 proton, Z would be . The true Z* for Li = 1.3 (Between the two possible values.) The concept of Effective Nuclear Charge will help us explain many of the trends that we see across the periodic table. Atomic Size The boundaries of an atom are fuzzy because the locations of the electrons are functions. Radius is assumed to be ½ the distance between the nuclei of two covalently bonded identical atoms. Picture from McMurry Fay Page 8-9 Trends in Atomic Size a) Size gets as you go __________ a family. (You are adding a new set of energy levels (shell) for each row you go down. b) Size gets across a period. as you go __________ (Not what you would first expect; You are adding more electrons which repel each other so you would expect the radius to . This is overshadowed by the increase in as you go across the table. (You are adding to the energy level so shielding from the new electrons is . However, the increased number of (+) charges in the nucleus attracts the electrons closer in. When you get to the end of a period or row, the size dramatically because you are now adding to a new and ALL of the previous electrons now provide shielding. Ionic Radii Cations Cations always have a radius that the parent atom. - The same number of (+) charges are now distributed between a ____________ number of (-) charges. (The electrons are held closely.) - There are e- to e- repulsive forces. - The larges change occurs when the e- removed is the last electron in the outer . Ca > Ca+ Ca >>> Ca 2+ [Ar] 4s2 [Ar] 4s1 [Ar] 4s2 [Ar] (The 4th energy level is no longer used.) Page 8-10 Example: The radius of Na = 154 pm; Na+ = 116 pm Picture from McMurry Fay Anions Anions always have a radius that the parent atom. - The same number of (+) charges are now distributed between a ____________ number of (-) charges. (The electrons are held closely.) - There are e- to e- repulsive forces. Example: The radius of Cl = 97 pm; The radius of Cl- = 167 pm Picture from McMurry Fay Isoelectronic Species Isoelectronic species are ones that have the same number of different numbers of . As the number of protons goes goes . , the attractive force goes Page 8-11 but and the radius Ionization Energy The ionization energy is the energy required to remove atom or ion in the state. electron from an 1st Ionization Energy Ei1 E is always X(g) X+(g) + 1 e- . 2nd Ionization Energy Ei2 E is always X+ (g) X2+(g) + 1 e- than Ei1. 3rd, 4th, 5th etc ionization energies can also be calculated. Example: 1st 2nd 3rd 4th Al(g) Al+(g) + 1 eAl+ (g) Al2+(g) + 1 eAl2+ (g) Al3+(g) + 1 eAl3+ (g) Al4+(g) + 1 e- I.E. (kJ/mol) 580 1,815 2,740 116,000 Electron Config. [Ne] 3s2 3p1 [Ne] 3s2 [Ne] 3s2 [Ne] 3s1 [Ne] 3s1 [Ne] [Ne] [He] 2s2 2p5 Why is the 4th I.E. so large? You are trying to remove an electron from a noble gas electron configuration which is very . Page 8-12 Trends in Ionization Energy a) I.E. as you go ________ across the periodic table. Zeff goes up as you go right (more positive charge and ineffective shielding because each new e- is in the same shell. It gets harder and harder to remove an electron that is held more tightly. b) I.E. as you go the group or family. You are removing electrons that are farther out in shells that are farther from the nucleus and are better screened from the pull of the nucleus. Anomolies in Ionization Energy Picture from McMurry Fay Unusually high I.E.s are observed for Be, N, Mg, and P. These high I.E.s for some elements can be explained as follows: Be/Mg: You are removing an e- from a filled (stable) orbital. N/P: You are removing an e- from a ½ filled (stable) sublevel. Elements with low ionization energies make good (They give up an electron relatively easily.) Page 8-13 agents. Electron Affinity Electron afinity is defined as the energy change that occurs when an electron is to the lowest energy unoccupied orbital in a atom. X(g) + 1 e- X-(g) Adding a second electron E can be _________________________ E is always due to repulsive forces. Examples: F(g) + e- F-(g) E.A. –328 kJ/mol N(g) + e- N-(g) E.A. +7 kJ/mol Ca(g) + e- Ca-(g) E.A. –2 kJ/mol Ne(g) + e- Ne-(g) E.A. +116 kJ/mol F becomes more stable (attains a noble gas configuration). N & Ca stabilities are not significantly changed. Ne becomes less stable (looses the noble gas configuration) Note: Our text assigns a negative energy to an EA that is favored and a positive energy to one that is not spontaneous to be consistent with the other energy related terms we have studied. Many other textsreverse the signs so that an atom that wants an electron has a high positive value. We need to realize that when we say that an element has a we are talking about it having a large Page 8-14 electronegativity, value!