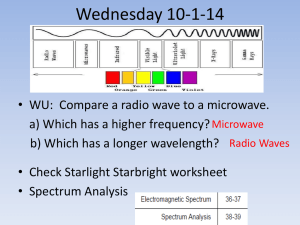
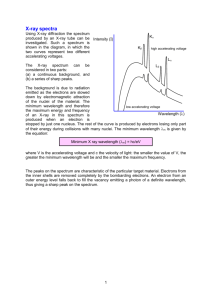
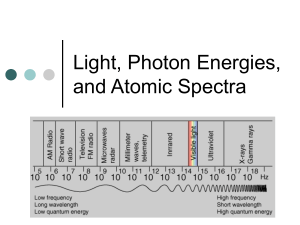
Spectrum Analysis
advertisement

Tutorial: Spectral Analysis In many respects, light exhibits a wave-like behavior. As with waves in water, this means light waves have a velocity (c in a vacuum; 300,000 km/s), a wavelength (lambda), and a frequency (nu). The distance a light wave travels in one second is its velocity, expressed in meters per second (m/s); the distance between two wave crests (or troughs) is the wavelength; the number of waves that pass per second is the frequency (number/second). Wavelength, frequency, and speed are related by the following equation: speed = wavelength x frequency ; or, c The energy and frequency of a specific photon emitted during a transition are in direct proportion: energy = constant x frequency; or, E h , where h is Planck’s constant. Read the appropriate sections of your text and lecture notes and answer the following questions: Continuous Spectra A rainbow is formed when rain drops break the Sun's light into the component colors. Light passing through a prism also forms a rainbow, but in this case we call it a spectrum (plural: spectra not spectrums). A spectrum of the Sun or a light bulb (both approximately behaving like blackbodies) will have all of the colors of the rainbow. These spectra are called _____________________________. Emission and Absorption Spectra 1. Each element produces a unique set of emission or absorption lines. An emission spectrum involves transitions of electrons from ___________ to ______________ energy states. An absorption spectrum involves transitions of electrons from _______________ to ___________ states. These transitions occur only between discrete energy levels, and thus the lines occur only at certain wavelengths and at no others. 2. For a given atom, will the discrete wavelengths we see be different depending on whether we are viewing an emission spectrum or an absorption spectrum? Explain why or why not. 2/6/16 687292843 tu 97 Consider just four of the energy levels in a certain atom, as shown in this diagram: a. Assume an emission spectrum and draw arrows indicating the possible transitions. b. How many spectral lines will result from all possible transitions among these levels? __________ c. Which transition corresponds to the greatest energy change? From n = _______ to n = ________ . d. Which transition corresponds to the smallest energy change? From n = _______ to n = ________ . The transition with the greatest energy change will result in a photon with the highest frequency and the shortest wavelength. The transition with the smallest energy change will result in a photon with the smallest frequency and the longest wavelength. This is because energy and frequency are directly proportional to each other. The wavelength of a photon is inversely proportional to its frequency, and therefore also inversely proportional to the energy. Make sure you understand how this all “works.” In this diagram containing 5 energy levels and therefore 10 possible transitions, the energy “difference” (in any units) between the states is given. Rank the transitions from that with the largest energy difference to that with the smallest energy difference. AE, BE, __________ , __________ , AD, BD, __________ , __________ , BC, __________ . If you were to rank these transitions in order of the ones producing the highest frequency photon to the lowest frequency photon, would you rank them in the same order or opposite order? Explain your answer. If you were to rank these transitions in order of the ones producing the longest wavelength to the shortest wavelength, would you rank them in the same order or opposite order. Think about this, and then explain your answer. Sometimes when we avoid the math altogether it is actually HARDER to understand a concept than if we included the math. Let’s see just how we would calculate the wavelengths we see from excited hydrogen atoms. tu 98 Pulling it all together: the spectrum of the hydrogen atom Let’s take some of the concepts introduced above and work with them so we get a better understanding. We will just be dealing with the hydrogen atom. It’s complicated enough, and the helium atom—with just 2 electrons—is so much more complicated that it would be impossible to describe here. We can use the Bohr model of the atom only for hydrogen. The above image shows a continuous spectrum (top), an absorption spectrum (middle), and an emission spectrum (bottom) for some of the “Balmer lines” of hydrogen—those that lie within the visible part of the spectrum. The sketch to the right is a more-detailed version of a figure given in your text, indicating additional possible transitions for an electron in a hydrogen atom. Notice that the energy jump of the electron from n = 3 to n = 2 results in a photon of wavelength 656.3 nm, or one that is in the red part of the spectrum. For an electron to jump from n = 2 to n = 3 would involve absorbing a photon of wavelength 656.3 nm. If the electron were at n = 2 and absorbed a photon having an energy of more than 3.4 electron volts (eV), it would have enough energy to escape the proton. If the proton were to capture a free electron, then a photon having a wavelength of 364.6 nm would be emitted. The line would be off to the left of the spectra shown above, deep in the violet part. Further explanation is given just below in these notes. First of all, let’s review the relationship between wavelength and frequency. Recall: c = . We know the speed of light, 2.9979 x 10^8 m/s, and so if we have either the wavelength or the frequency, we can calculate the other value. For example (and let’s stick with those electron jumps that result in photons in the visible part of the spectrum for now), the jump from energy level 3 to 2 results in a line at 656.3 nm. What is the frequency of this photon? How does the frequency of this photon compare to that of a photon with a wavelength of 364.6 nm? tu 99 c wavelength frequency For a wavelengt h of 656.3 nm, or 656.3 10-9 m or 6.563 10- 7 m (in scientific notation) : frequency c 2.9979 108 m/s 4.57 1014 per second (Hz) 7 wavelength 6.563 10 m For a wavelengt h of 364.6 nm (3.646 10- 7 m) : frequency 2.9979 108 m/s 8.22 1014 per second (Hz) 7 3.646 10 m In this comparison, we see that the photon with the shorter wavelength has a higher frequency; that is, more waves are going to pass per second by any given reference point. Note that the energy jump that resulted in the shorter wavelength was greater (more than 3.4 eVs) that the one with the wavelength of 656.3 nm (about 1.9 eV’s). Do you see a pattern forming here? A wavelength of 656.3 nm is about 1.8 times 364.6 nm; the ratio of the frequencies (higher divided by lower) is about 1.8; 1.9 eVs times 1.8 is 3.4 eVs. They are all related. You could do the same kind of quantitative analysis for each of the other wavelengths for the hydrogen lines. It does not matter whether these lines are seen in absorption, with the electrons absorbing photons of specific energies, or emission, with the electrons emitting photons of specific energies: it is the ratio of the energy jumps that is important. Since we learned above that the energy of a photon is directly proportional to its frequency, we also now know that the photon with the shorter wavelength also has more energy. How do the energies actually compare? The energy is equal to Planck’s constant times the frequency, E h . Planck’s constant is energies using the frequencies calculated above we find: 6.626 1034 joule sec . Comparing E (6.626 1034 joule sec)(4.57 1014 /second ) 3.03 1019 joules 1.9 eVs E (6.626 1034 joule sec)(8.22 1014 /second ) 5.45 1019 joules 3.4 eVs where we’ve used the fact that 1 eV 1.602 10 joules (the “seconds” units cancel). Do you see how all of this is related? Try to follow the general steps taken in this mathematical analysis even if you do not understand it all. If we were to do similar calculations for the electron jumps that started or ended with n = 1, what is called the Lyman series, we would find out that these are photons with a great deal of energy. Since we see that they have shorter wavelengths, they must have higher frequencies, and higher frequencies mean higher energies. We can relate these to something more familiar to us: the ultraviolet radiation we receive from the Sun that causes premature aging and even cancer. UVA photons have wavelengths between 320 and 400 nm; UVB, 280 – 320 nm; and UVC, 180 – 280 nm. UVC radiation is extremely toxic to life. Fortunately for us, the Earth has developed an ozone layer that blocks this harmful radiation. -19 It is important to note that the hydrogen atom has these signature lines—this unique fingerprint—no matter what the temperature of the star is. For stars that are moving towards us or away from us, the wavelengths that we observe will be slightly different, but the pattern of lines will be the same. If we correct for the motion of the star, we will find that the wavelengths are exactly those that we measure in a laboratory here on Earth. Exactly. Without exception. The same can be said for every other element, for the ion of those elements, or for the isotopes of those elements. In each case, we see a unique fingerprint that identifies the element, ion, or isotope. Now, stars in real life are not made up of pure hydrogen, they all have helium, with a dash of carbon, nitrogen, oxygen, and other elements mixed in. What we observe, then, are these unique spectra all piled on top of each other. Unraveling the composition of the star is much like tracking down a criminal when there are 20 or 30 different sets of fingerprints on the weapon. Astronomers meticulously, with the help of computers (naturally) unravel the elements present in a star by matching the patterns, element by element, until as many as possible are identified. tu 100 Digital versus Photographic Spectra When we showed the continuous, emission, and absorption spectra above we were viewing how these spectra would look if the spectrum of a star were recorded on a photographic plate. Just like film in a camera, these plates were sensitive to light and brightened where the light was the most intense, and reacted very little where there was not much light being received. Thus the term “lines” arose when talking about emission and absorption. Today’s detectors are digital devices—CCDs—that record the spectrum in two dimensions: Relative flux (brightness) versus wavelength. As you will probably note from the comparisons given for two stars having very different surface temperatures, we are able to get a lot more information from the digital spectrum than from the photographic spectrum (usually the photographic spectra were in black and white NOT color). Compare the two spectra in each of the two images figures that follow. Do you see the dark lines that are in exactly the same places where the relative flux takes a “dip”? In the first figure for the A star, the violet end of the spectrum is much brighter than the red end. In the K star, no violet light is detected, and the star is the brightest in the green-yellow part of the spectrum. What we are looking at is the underlying continuous spectrum of each star, the spectrum that contains information about the star’s temperature. Which star will look “bluer”? Which star will look “redder”? Which star is the hotter? Which star is the cooler? There is more accurate information about the star’s surface temperature in the lines that we see – the depths of the lines and which ones are the strongest. We will need to wait to understand this part of stellar astronomy . We need much more information about the spectra of stars – their “spectral types” – than we’ve had time to study so far. tu 101 This page blank. tu 102