solutions
advertisement

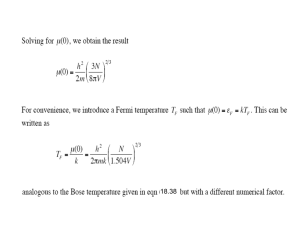
19.2) consider a kilomole of 3 He gas atoms under STP conditions No. of 3 He atoms at STP = N = 1 kilomole = 6.023 10 26 Volume at STP =V = 22.4 dm 3 Mass of 3 He atom = m = 3 27 4 . 98 10 6.023 10 26 a) What is the Fermi temperature of the gas? Fermi Temperature = TF ? We know that h2 N TF 2mk 1.504v 2 3 6.626 10 6.023 10 26 2 4.98 10 27 1.38 10 23 1.504 22.4 0.069 K 0.07 K Fermi Temperature TF 0.07 K 34 2 b) Calculate kT and exp kT 2 3 ? kT we knowthat 2mkT 3 2 V ln 2 2 kT N h 3 27 23 2 2 4 . 98 10 1 . 38 10 273 22.4 ln 2 26 34 2 6 . 023 10 6.626 10 kT 12.7 exp e kT e kT kT ? 3.28 10 5 c) Find the average occupancy f of a single particle state that has energy of 3 kT 2 Energy : 3 kT 2 Chemical Potential : 12.7kT Average Occupancy : f ? We knowthat f e 1 KT 1 1 3 12.7 kT 2 kT e 1 1 1.469 10 6 f 6.8 10 7 Average Occupancy f 6.8 10 7 19.3) For a system of noninteracting electrons, show that the probability f of finding an electron in a state with energy ∆ above the chemical potential μ is the same as the probability of finding an electron absent from the with energy ∆ below μ at any given temperature T N 1 g e kT 1 where 1 f e kT 1 for the probability of absent at 1 1 1 f 1 e kT 1 e kT 1 f 1 f e kT 1 1 1 1 1 1 e kT e kT therefore they are equal to each other e kT 19.4a) Verify that the average energy per fermion is a direct calculation of U 0 Use equation 19.18 35 F at absolute zero by making N 3 U N F for T 0 5 U 3 T o F N 5 b) Similarly, prove that the average speed of a fermion gas particle at T=0 is where the Fermi velocity VF v f v g v dv 0 N v is defined by 2 F 3 5 mvF 3 4 VF , f v N v g v 1 1 2 mv 2 kT e 1 g d g v dv 8 2V 3 2 1 2 2 1 g v dv m mv mv dv h3 2 2 1 1 1 2 2 1 mv mv dv 8 2V 3 2 2 2 v m 1 3 mv 0 h 2 kT e 1 2 8 2V v h3 3 1 2 3 m 0 2 v3 1 2 mv 2 kT dv e 1 need to use int egration in part ! 19.6) At very low temperature, the electronic specific heat capacity of a metal is approximately ce AT where A can be determined by experiment. For gold, the constant A is found to be A 0.73J kilomole K . Compare this to the value obtained from Equation (19.20). (The atomic weight of gold is 197 and its 3 3 density is18.9 10 kgm ) 1 1 From eqn (19.20), one identifies 2 Nk 2 TF 1 1 6.63 1034 3 18.9 103 with TF F 6.02 1026 5 1 31 k 8.62 10 eVk 2 9.11 10 8 197 TF 62028k 2 A 2 6.02 1026 1.381 1023 Jk 1 2 62028k 1 0.66 J kilomole K 2 19.7 a) Calculate F for aluminum assuming three electrons per aluminum atom. 3 Kg N 2.69 10 m 3 6.02 10 26 atoms 5.99 10 28 V kilo mole 27 Kg kilomole N # Density for electrons 3 V 1.8 10 29 F 6.63 10 3 1.8 10 29 2 9.1110 31 8 34 2 b) Show that the aluminum at T=100 K, μ differs from aluminum is 2.69 10 kgm 3 3 2 3 5.6 2.1 11.8eV F by less than 0.01%. (The density of and its atomic weight is 27.) 2 3 2 0 1 12 T TF 2 2 1000k 0 1 11.8eV 12 8.62 10 5 eVk 1 0 1 4.38 10 5 2 less than 0.01% c) Calculate the electronic contribution to the specific heat capacity of aluminum at room temperature and compare it to 3R T 2 kT Nk Nk 2 TF 2 F 8.617 10 5 eVk 1 2.43 10 3 3 n R 2 1 297 J kilomole k Ce 19.8) In sodium there are approximately 2.6 10 conduction electrons per cubic meter, which behave as a free electron gas. Give an approximate value for the electronic specific heat of sodium at room temperature. (The atomic weight of sodium is 23.) In the series expansion for c e , show that at this temperature the cubic term is negligible compared with the linear term. 26 N 2.6 1028 V 6.63 10 28 3 2.6 10 F 2 9.11 1031 8 5.6eV 0.579 3.243eV 34 2 2 3 k 298 Ce Nk R 0.008 2 3.243 2 325J kilomole1k 1 2 19.10) calculate the isothermal compressibility of the fermion gas consisting of the free electrons in silver. Compare your answer with the experimental value for silver of 0.99 1011 Pa 1 1 2v V 2 p T from eq 19.24 P 2 NkTF 5 V 2 h2 N 3 1 TF 2mk 1.504 V 2 2 3 eq 19.8 2 h2 N 3 1 P Nk 5 2mk 1.504 V 2 Nh 2 N 3 1 P 5 5m 1.504 V 3 5 3 2 V 5 3 Nh 2 N 3 1 5m 1.504 p 3 Nh 2 N 3 5 1 V 35 5 m 1 . 504 p 2 3 5 dv Nh 2 N 3 3 58 P dp 5m 1.504 5 2 3 5 1 Nh 2 N 3 3 58 P v 5m 1.504 5 3 this equation leads to P with P 2.1 1010 Pa from textbook 5 3 3 P 2.1 1010 Pa 1.26 1011 Pa 1 5 5 2 19.12) For the white dwarf star Sirius B, do the computation leading to Rmin 7 10 6 m Use Equation (19.28) and (19.30) with the appropriate parameter value. Rmin 7 10 6 m We knowthat 2a 19.32 b calculatin g b Rmin 3 b GM 2 19.30 5 G Gravitational energy 6.673 10 11 M Mass of the sun 2.09 10 30 kg 3 b 6.673 10 11 2.09 10 30 5 b 1.75 10 50 2 calculatin g a 2 3h 2 9 3 5 3 a N 10me 32 2 calculatin g N 5 1.82 10 22 7.23 10 20 6.10 10 56 2 5.33 10 14 so value of a is N 2 2 3 6.626 10 34 9 3 a 6.10 10 56 31 2 10 9.1 10 32 a 1.45 10 37 0.0933 4.388 10 94 a 5.92 10 56 put values of a and b in 19.32 Rmin 2 5.92 10 56 1.75 10 50 6.77 10 6 7 10 6 Rmin 5 3 19.13) consider the collapse of the sun into a white dwarf. For the sun, M= 2 10 R= 7 10 8 30 kg m , V=1.4 1027 m3 a) Calculate the Fermi energy of the sun’s electrons No. of electrons 2 10 30 1.205 10 57 1.66 10 27 1 of nucleous 2 N 1.205 2 10 57 V 1.4 10 27 # of electrons h2 F 2me 3N 8V 2 3 1.205 7.23 10 27 F 0.33me v 27 1 . 26 1 . 4 10 5 F 0.33me v 6.248 10 2 3 F 20.6eV b) What is the Fermi Temperature? TF F k 20.6eV 2.39 105 k 5 1 8.62 10 eVk c) What is the average speed of the electrons in the fermion gas (see problem 19-4). Compare your answer with the speed of light. N 1.205 2 1057 31 Density 9.11 10 9.11 10 V 1.4 1027 0.39 kg 3 m 31