Chapter 7 lecture 2
advertisement

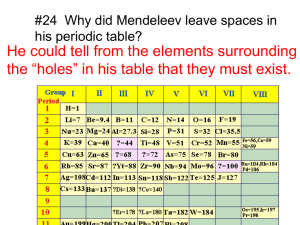
Secs 7.3-8: Periodic Properties Review: electron configurations and effective nuclear charge Goal: relate metallic/nonmetallic character of elements to position on the Table in terms of electron configuration and Zeff We will focus on 3 properties: Atomic/ionic radii Ionization energy Electron affinity 134 Atomic and ionic radii How to accurately determine the radius of an atom? Use an alternate definition: e.g., for N2: treat the N-N bond length as the sum of atomic radii of 2 N atoms to give the bonding atomic radius What are the periodic trends in bonding atomic radii? note that: Li > Be > B > C > N > O > F Na > Mg > Al > Si > P > S > Cl (Check out Fig 7.6...) In general: within each group: atomic radius increases from top to bottom within each period: atomic radius decreases from left to right 135 What factors determine the atomic radius? what determines the size of the outermost orbital? Orbital size is determined by: Principal quantum number Effective nuclear charge Increase n: increase orbital size Increase Zeff: decrease orbital size Moving across a period: filling orbital with same n value; what happens to Zeff? Moving down a group: changing n value of outermost orbital; what happens to Zeff? e.g., arrange the following atoms in order of increasing atomic radius: Ca, Mg, Be P, Cl, Sr 136 Which has a larger effect on radius: moving one place across a period or moving down one group? Why? Sizes of Ions Very important in determining structure/stability of ionic compounds Why? Recall force of attraction between charges: F kQ1Q2 d2 How does this affect the attractive force between ions in a compound? Ionic size depends on: nuclear charge # of e- in the ion orbitals in which outermost e- reside 137 In general, cations are smaller than their parent atoms anions are larger than their parent atoms Periodic trends in ionic size for ions of the same charge, size increases moving down a group isoelectronic series: ions with same # of ee.g., O2-, F-, Na+, Mg2+, Al3+ what is the trend in size in this series? why? e.g., arrange the following atoms & ions in order of increasing size: Ar, Cl-, S2-, K+ 138 Electron configurations of ions When a cation or anion is formed, e- are added/removed from the orbital with the largest value of n: E.g., write electron configurations for Na+, S2- Ionization of transition-metal ions different than representative elements most transition metal atoms do not ionize to noblegas configurations transition metal atoms lose their valence shell s efirst, followed by d e- e.g., write the electron configurations for Cr3+ Co2+ V2+ 139 Ionization energies The first ionization energy I1 is defined (in the gas phase) by the reaction M(g) M(g)+ + e- The second ionization energy I2 is defined by M(g)+ M(g)2+ + elarger value of I: stronger binding of e- to atom or ion Removal of an electron always requires energy! Consider the following ionization energies (kJ/mol): element I1 I2 I3 Na 496 4560 Mg 738 1450 7730 Al 577 1816 2744 Removal of successive e- requires more energy: why? 140 What happens to I after a noble-gas configuration is reached? Periodic trends in ionization energies Within each period: I1 generally increases with atomic number alkali metals: lowest I.E. noble gases: highest I.E. Within each group: I.E. decreases with increasing atomic number How to explain these trends? 141 I.E. depends on both Zeff and distance from e- to nucleus moving left to right: Zeff increases, atomic radius decreases moving down: Zeff constant, atomic radius increases E.g., Based on position on the periodic table, which of the following pairs would have the largest first ionization energy? Si, C K, Cr 142 Electron affinity ionization: M(g) M(g)+ + emeasures energy changes associated with removing e- electron affinity, E: M(g) + e- M-(g) measures energy changes that occur when e- is added to atom or ion Sign convention for electron affinity (EA) For cations and most neutral atoms: adding e- is exothermic; EA is generally negative For anions and some neutral atoms: EA positive (work must be done to force e- onto atom) Notice that.... Groups 2A, 8A: E is positive Group 7A (halogens): E is strongly negative why? 143 Periodic trends in electron affinities In general, EA becomes more negative as we move across a period 'exceptions': atoms with filled or half-filled subshells, e.g., Be, N, Ne Moving down a group: EA does not change greatly why? Periodic properties of groups of atoms Three broad catagories of elements Metals Semimetals Nonmetals What are the properties of these groups? What differentiates them? 144 Metals Properties Metals tend to have low ionization energies and tend to form cations easily (easily oxidized) They tend to be large in size They tend to have only slightly negative electron affinities We will use these three properties - low IE, large size, slightly negative EA – to define metallic character What is the periodic trend in metallic character? Where are the most metallic elements? The least metallic? 145 General trends in reactivity of metals Metals react with nonmetals to form solid ionic compounds (salts): M(s) + NM(g, l) ionic compound(s) A particularly important reaction of this type is between a metal and oxygen to form a metal oxide (MO), e.g. 4Fe(s) + 3O2(g) 2Fe2O3(s) Metal oxides are basic, ionic solids; those that dissolve in H2O form metal hydroxides (MOH), e.g., MO(s) + H2O(l) MOH(aq) e.g., write a balanced equation for the reaction of iron (III) oxide with water. 146 Metal oxides react with acids to form salts and H2O: MO(s) + acid(aq) salt(aq) + H2O(l) e.g., write a balanced equation for the reaction of nickel (II) oxide with aqueous hydrochloric acid Active metals (e.g. groups 1A and 2A) react with water to form MOH and hydrogen: M(s) + H2O(l) MOH(aq) + H2(g) E.g., write a balanced equation for the reaction between solid potassium and water. 147 Active metals will also react with aqueous acid to form a salt and hydrogen gas (cf. activity series) M(s) + acid(aq) salt(aq) + H2(g) E.g., write a balanced equation for the reaction between solid aluminum and nitric acid. Summary: Metals are characterized by: _______ ionization energies _______ size _______ electron affinities Metallic character ____________ moving right to left across a period and ___________ moving down a group. 148 Metals react with nonmetals to form ____________ Metal oxides are _________ oxides. Metal oxides react with acids to form _______ and _______. Active metals react with water to form __________ and _________. Active metals react with acids to form ___________ and _________. Notice: the more metallic an element, the more basic its oxide and the more reactive the element will be towards acid and water! 149 Group trends for the active metals Group 1A: alkali metals: Lowest I1 of all the elements Exist in nature only as compounds Form solid hydrides, sulfides, and chlorides Form oxides/peroxides/superoxides in reactions with oxygen Group 2A: alkaline earth metals Less reactive than alkali metals (higher I1) Lighter species (Be, Mg): do not react with H2O(l) Heavier species (Ca, Sr, Ba) react readily with H2O(l) 150 Nonmetals Properties Tend to have very large ionization energies Tend to be small in size Tend to have very negative electron affinities – gain electrons and are easily reduced We will use these three properties - high IE, small size, very negative EA – to define nonmetallic character What is the periodic trend in nonmetallic character? Where are the most nonmetallic elements? The least nonmetallic? 151 Reactions of nonmetals tend to form anions in reactions with metals: Metal(s) + Nonmetal(g,l) salt(s) Nonmetal oxides (NMO) are acidic oxides: dissolve in H2O to form acids NMO(g,s) + H2O(l) acid(aq) e.g., write a balanced equation for the reaction of carbon dioxide with water. Summary: Nonmetals are characterized by: _______ ionization energies _______ size _______ electron affinities 152 Nonmetallic character ____________ moving left to right across a period and ___________ moving up a group Nonmetal oxides are ____________ oxides. Notice: the more nonmetallic an element, the more acidic its oxide! E.g., Arrange the following oxides in order of increasing acidity: CO2, MgO, Al2O3, SO3, BaO, P2O5 153 Group Trends for Nonmetals Hydrogen Forms +1 ion with nonmetals Forms -1 ion with active metals – much higher ionization energy than metals Forms solid hydrides with metals Group 6A: Oxygen: two forms (or allotropes) Molecular oxygen, O2 Ozone, O3 Generally forms oxide ion Also forms peroxide ion O22- and superoxide ion O2Sulfur Exists as 8-membered rings; brittle molecular solid (usually just write S(s) rather than S8(s)) 154 Group 7A: halogens all are typical nonmetals consist of diatomic molecules: F2(g), Cl2(g), Br2(l), I2(s) EA is the most negative of the elements; tend to form halide anions Group 8A: Noble gases Exist as monoatomic gases in nature 155 Problems du Jour Arrange the following atoms in order of increasing atomic radius: Mg, F, P, O, Ca In, Ar, Rb, Ge, He Why is the second ionization energy of K much greater than its first ionization energy? 156 Problems du Jour Write balanced equation for the following reactions: Lithium with water Calcium oxide with water Copper (II) oxide with nitric acid Aluminum with chlorine gas Sulfur with oxygen Sulfur trioxide with water 157