Chapter 7: Periodic Properties of the Elements
advertisement
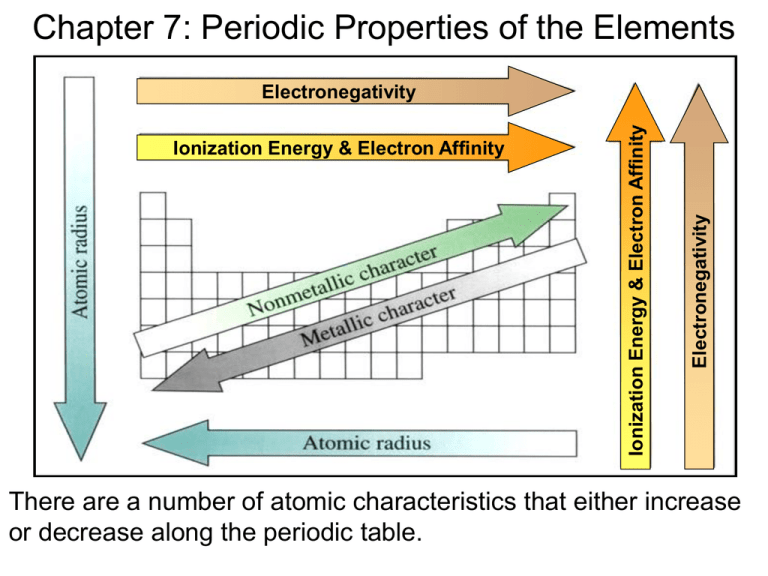
Chapter 7: Periodic Properties of the Elements Electronegativity Ionization Energy & Electron Affinity Ionization Energy & Electron Affinity Electronegativity There are a number of atomic characteristics that either increase or decrease along the periodic table. Atomic Radius: As you go down a group, the atomic radius increases with increasing energy level. Atomic radius decreases as you go left to right along a period because the greater nuclear charge pulls the electrons in closer to the nucleus. s-block p-block Why ionization energy decreases and atomic/ionic radius increases as go down a group: Shielding effect: The inner electron shells insulate the valence electrons from some of the electrical attraction with the positive charge of the nucleus. + valence enucleus core electrons Why ionization energy increases and atomic/ionic radius decreases as go across a period: Increasing Effective Nuclear charge(Zeff): Electrons in the outermost energy levels do not effectively screen each other from an increasingly positive nucleus. nucleus - - - - + + + + + + + Zeff = Z - S Li: Zeff = 3 – 2 = 1 N: Zeff = 7 – 2 = 5 A few definitions: van der Waals radius: The nonbonding radius of atoms Covalent radius: Isoelectronic: The radius of atoms covalently bonded to another atom; is smaller than the van der Waals radius Different ions that have the same number of electrons Ex: O2-, F-, Na+, Mg2+, Al3+ all have 10 electrons Ionization energy: The amount of energy required to remove an electron from an atom or ion. Electron affinity: The amount of energy released when an electron is added to an atom or ion. There is a spike in ionization energy whenever a noble gas electron configuration is disrupted. Q: What is the valence electron configuration for an atom that has the following ionization energies: 1st: 734 kJ/mol, 2nd: 1850 kJ/mol, 3rd: 16,432 kJ/mol A: ns2 Large spike in IE indicates noble gas core is disrupted Electron Affinity: the ability of an atom to gain an electron. This is closely related to ionization energy, and increases going left to right, and decreases going down. Electronegativity: Ability of an atom to attract electrons when in a molecule. Electronegativity increases going left to right, and decreases going down. Electron configurations of ions •If electrons are added to make an anion, they fill the lowest energy levels first. (Auf bau principle) •If electrons are removed to make a cation, the are taken from the highest energy levels first. Atom Li 1s2 2s1 Ion 1s2 2s0 Li+ Fe [Ar] 4s2 3d6 [Ar] 4s0 3d6 Fe2+ [Ar] 4s0 3d5 Fe3+ Fe3+: 4s 3d Notice that the Fe3+ ion has the maximum multiplicity possible. Hence, its greater stability wrt Fe2+ Electron configuration exceptions and their ions Atom Cr Cu Au [Ar] 4s1 3d5 [Ar] 4s1 3d10 [Xe] 6s1 4f14 5d10 Ion [Ar] 4s0 3d5 Cr+ [Ar] 4s0 3d3 Cr3+ [Ar] 4s0 3d0 Cr6+ [Ar] 4s0 3d10 Cu+ [Ar] 4s0 3d9 Cu2+ [Xe] 6s0 4f14 5d10 Au+ [Xe] 6s0 4f14 5d8 Au3+ Characteristic Properties of Metals and Nonmetals Metals Metalloids • Have a shiny luster and are usually silvery in color Notable exceptions: Cu Au Nonmetals • Do not have luster; various colors • Solids are usually brittle • Solids are malleable • Poor conductors of heat and ductile Hg is only liquid metal at RT and electricity • Good conductors of heat and electricity • Metal oxides are ionic solids and form basic solutions • Form cations in solution • Most nonmetal oxides are molecular solids that form acidic solutions. • Tend to form anions or oxyanions in solution Elements with Color Pale yellow gas Light green gas Dark orange liquid/gas Dark violet crystals/gas Properties of SOLID ionic compounds (M + NM) • Poor conductors of electricity and heat. • Generally high melting (more than 150°C). • Crystalline, hard and brittle. NaCl crystal lattice structure Properties of ionic compounds (cont.) • Molten ionic compounds form liquids that are an electrical conductors. • Ionic solids that are water soluble, dissolve to form solutions that are electrical conductors. • The solubility of ionic compounds depends upon the lattice energy. The greater the lattice energy, the lower the solubility. + - Acid-Base Behavior of Oxides • Most metal oxides form basic solutions • Most nonmetal oxides form acidic solutions • The acidity of the solution increases with oxidation number of the central atom pH: SO3(aq) < SO2(aq) Hint: an easy way to evaluate the acidity is that pH as the # oxygen atoms • Amphoteric substances can act as either an acid or a base Group Trends Alkali metals: low IE highly reactive, soft silvery metals with a low density and low melting point. While lithium reacts with oxygen to form lithium oxide 4 Li + O2 2 Li2O the other alkali metals form peroxides. (peroxide = O22-) 2 Na + O2 Na2O2 Potassium, rubidium and cesium react with oxygen to form superoxides (superoxide = O2) K + O2 KO2 Alkaline earth metals • Harder, more dense and have a higher melting point than Group 1 metals. • 1st IE is low, but not as low as Group 1 because disrupting pseudo-noble gas configuration (s2) • Increasing reactivity with increasing atomic number due to increase in nuclear shielding. • Beryllium will not react with water, but the other alkaline earth metals will to form the metal hydroxide and hydrogen gas. Ca + 2 H2O Ca(OH)2 + H2











