BERM Center and PKD Core Center FRET and Imaging Microscopy
advertisement

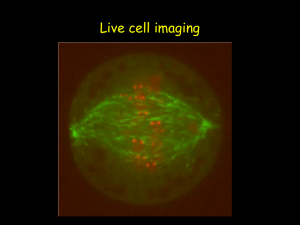
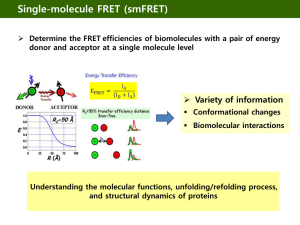
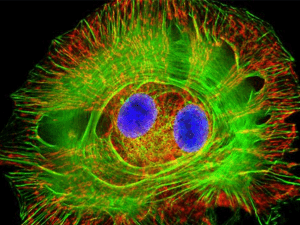
BERM Center and PKD Core Center FRET and Imaging Microscopy Shared Core Facility Director: Joanne E. Murphy-Ullrich, PhD Department/Center Association: BioMatrix Engineering and Regenerative Medicine (BERM) Center The facility is supported by the BERM Center and the High Resolution Imaging Facility. The FRET Microscopy Core is partially supported by the UAB Recessive Polycystic Kidney Disease Core Center P30 grant. Mission The mission of the FRET and Imaging Microscopy Shared Core Facility is to provide biomedical researchers at UAB with state-of-the art instrumentation combined with expert technical support to enable them to visualize molecular interactions in living cells, to assist with image analysis, and to aid in data interpretation. These capabilities provide state-of-the-art facilities for advanced cell imaging, time lapse, and optical sectioning applications. A goal of this Core is to provide training for the UAB research community in this technology, as well as to foster new research initiatives and collaborations. Definition Fluorescence resonance energy transfer (FRET) microscopy is a powerful approach to reveal intermolecular interactions in live and fixed cells. The energy transfer is directed from higher-energy donor fluorochrome to lower-energy acceptor fluorochrome of labeled protein pairs. Target protein pairs are likely to exhibit FRET if they are labeled with spectrally overlapping donor-acceptor fluorochromes and if they are within 10 nm apart. FRET imaging in our facility can be performed by widefield fluorescence microscopy or confocal microscopy. Advantage Research is presently limited to static approaches to understanding complex molecular events in cells through either biochemical analyses of cell extracts or immunolocalization of molecules on fixed cells. With FRET, specific intermolecular interactions can be observed with precise localization (10 nm or less) throughout various cellular compartments and organelles. There has been a resurgence of interest in FRET microscopy in recent years with the development of novel green fluorescent protein (GFP) and red fluorescent protein (RFP) variants and homologs -- CFP and Cerulean, YFP and Venus, m-RFP and m-cherry. The spectral and molecular properties of these tags afford more efficient FRET and reduced disturbance of native structure and targeting of proteins. FRET microscopy has broad applicability to studying intracellular cell signaling, studies of molecular complex formation such as receptor oligomerization, transcriptional studies, and analysis of membrane microdomains. Acceptor Photobleaching Direct visual proof of FRET in labeled cells can be obtained by bleaching a region of the acceptor and imaging the corresponding increase in fluorescence of the donor in that region. This occurs because the energy of donor is no longer transferred in the place where the acceptor has been effectively destroyed. Sensitized Emission FRET FRET can be demonstrated by selectively exciting the donor and exclusively imaging the acceptor. However, due to spectral bleed through between donor and acceptor emission channels, real FRET can only be confirmed after background subtraction. Appropriate background subtraction is determined from a series of control images involving donor-only, acceptor-only, and donor + acceptor FRET samples [see below]. As sensitized emission FRET is non-destructive, it lends well to time lapse imaging of live cells. This method can provide data on the agonist-dependent kinetics of interacting proteins. Ratiometric FRET Protein-protein interactions in live cells which are dependent on agonist or antagonist molecules can be examined by time lapse imaging of the FRET emission [excite donor, image acceptor] with respect to donor fluorescence [excite donor, image donor]. In this case, the FRET emission [numerator] and donor fluorescence [denominator] are inversely proportional, yielding more obvious fractional differences between periods of increasing and decreasing FRET than with non-ratiometric FRET analysis. This method therefore provides for heightened detection of FRET dynamics. High-resolution ratiometric FRET is best performed on High Resolution Imaging Facility inverted Leica SP1 confocal [Shelby site] with cells grown on glass bottom dishes. This confocal is equipped with 458nm/488nm laser lines and TK465nm/RSP500nm dichroic mirrors for adequate imaging of YFP FRET emission and CFP donor fluorescence. Ratiometric FRET can also be performed on this confocal system with GFP/m-cherry and Cy3/Cy5 FRET pairs. FRET by Widefield Microscopy The BERM FRET and Imaging Microscopy Core Facility is located in Shelby Room 135A. It includes the following instrumentation: a Nikon TE2000U inverted microscope, Fluorescence optics (Plan APO and Fluor objectives), the Optical Insights Dual View, a Roper CoolSnap HQ camera, Universal Imaging Corporation Metamorph Premiere software, a Sutter Lambda DG-5 light source with a Piezo filter changer, and Leica Confocal SP2 & SP1 Microscope Systems FRET upgrades. This facility is designed for all FRET methods including the acquisition of time lapse series of images with the options to directly measure the amount of FRET inside selected regions of interest (ROI). The results may be displayed as time dependent curves for each selected ROI. In addition, color encoded images may be produced for each time point. The Nikon TE2000 has a Noise Terminator mechanism in the light path which deviates stray light out of the light collection path, vastly improving the optical signal to noise ratio. The CoolSnap HQ camera is a 12 Bit system, which utilizes the Sony ICX285 progressive scan CCD with a quantum efficiency of greater than 60% making it ideal for FRET, FLIM, TIRF, FRAP and other fluorescent microscopy applications. The Dual View by Optical Insights allows for simultaneous viewing of excitation and emission of both the donor and accepter molecules. This capability translates into faster image acquisition times. The Lambda DG-4 by Sutter Instruments has piezo motors in it that provide microsecond shifts between excitation filters. With the combination of the Dual View and the DG-4, interaction or FRET between donor and acceptor can be determined with much greater accuracy than conventional filter wheels. The BERM system is set up for CFP/YFP FRET and also GFP/mRFP1 FRET. For convenient data analysis, we have Precision FRET Software from Circusoft Instrumentation and Metamorph Software from Universal Imaging. Recent Facility Upgrades In early 2006, we purchased additional instrumentation to further upgrade the facilities. The equipment upgrade for the FRET facility includes: an XY motorized stage, 100 x objective for the confocal FRET, Chromatek Filters, Software Journal upgrades, and an additional XP workstation. These upgrades will provide users with the ability to perform advanced techniques in FRET, cell tracking, image stitching, high definition tissue and cell analysis at higher magnifications, greater CFP/YFP visualization, and enhanced XYZ cell deconvolution. These additional capabilities make the Nikon Eclipse system particularly attractive for advanced cell imaging, time lapse, and optical sectioning applications. This will benefit a variety of users who are interested in advanced FRET, calcium imaging, 3-D cellular reconstruction, and multi-field live cell imaging. FRET by Confocal Microscopy Our Leica SP2 confocal system is outfitted to a Leica DMRXE water immersion microscope with laser lines, dichroic mirrors and software modules specifically designed for FRET. The software modules support FRET by acceptor photobleaching, sensitized emission, and ratiometric analysis. A wide selection of donor-acceptor pairs can be used, including CFP/YFP, GFP/m-cherry, and Cy3/Cy5. Our Argon laser produces excitation lines at 458 nm and 514 nm for respective excitation of CFP [donor] and YFP [acceptor]. The 458nm/514nm double dichroic mirror is ideal for reflecting the laser light for efficient excitation of CFP/YFP and for transmission of the resulting CFP/YFP emissions to the confocal scanner detector. The emission bandpass windows for CFP and YFP are variably set with our patented prism spectrophotometer to minimize channel bleed through -- 450-500 nm for CFP and 525-600 nm for YFP. For GFP/m-cherry FRET, the 488nm Argon laser line/RSP500nm dichroic mirror and 561nm solid state laser line/DD488nm-568nm dichroic mirror are respectively used. Cy3/Cy5 FRET is respectively performed with the 561nm solid state laser line/DD488nm-568nm dichroic mirror and 633nm He-Ne laser line/TD488nm-568nm-633nm dichroic mirror. The emission bandpass windows are set accordingly: 500-550nm for GFP, 575-650nm for m-cherry, 575620nm for Cy3, and 645-750nm for Cy5. The proprietary Leica confocal software provides for efficient analysis of FRET data. High-resolution ratiometric FRET is best performed on High Resolution Imaging Facility inverted Leica SP1 confocal [Shelby site] with cells grown on glass bottom dishes. This confocal is equipped with 458nm/488nm laser lines and TK465nm/RSP500nm dichroic mirrors for adequate imaging of YFP FRET emission and CFP donor fluorescence. Ratiometric FRET can also be performed on this confocal system with GFP/m-cherry and Cy3/Cy5 FRET pairs. Training Opportunities The FRET microscopy facility is staffed by personnel from the High Resolution Imaging Facility who have received training in FRET microscopy. Personnel from Universal Imaging (Metamorph) and Nikon provide periodic training in advanced imaging techniques. In addition, the BERM Center provides yearly workshop scholarships for a limited number of investigators to attend either the FRET training or workshops at other institutions. Users will receive one hour of free training and consultation. Additional time will be billed at $15.00 per hour. Contact Information Co-Director: Joanne Murphy-Ullrich, PhD Email: murphy@path.uab.edu Phone: 205-934-0415 or 205-975-9369 Technical Support: Shawn Williams or Albert Tousson Email: tzaron@uab.edu Phone: 205-934-7403; 205-934-7855 Approved by: Joanne Murphy-Ullrich, PhD, Co-Director Date: February 14, 2007 Click here to go back to the list of Core Facilities.