Product Design Specifications 2
advertisement

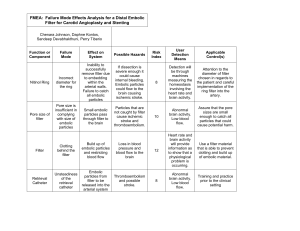
BioE 1160 Senior Design 2004-05 Bioengineering Department University of Pittsburgh, PA Number: Title: 001 Standard Controlled Document Form Doc. No. DJKT-001 Rev. 2 Date: 04/19/05 Status: Draft Revision: 2 Product Design Specification UNCONTROLLED DOCUMENT UNLESS ISSUED WITH A RED STAMP This document is confidential property of DJKT and may not be reproduced without prior written consent. Revision Approvals: Date: Originator Sandeep Devabhakthuni 04/19/05 [Originating dept. approval] Chenara Johnson 04/19/05 [Other dept. approval] Daphne Kontos 04/19/05 Quality Assurance Perry Tiberio 04/19/05 1.0 Purpose 1.1. State the specifications of the distal protection filter. 2.0 Scope 2.1. The re-design of the filter will be based on the devices requirements and uses. 3.0 References 3.1 N/A 4.0 Revision History 4.1. Revision 1: Proper document formatting, 11/17/04 4.2. Revision 2: Target Cost, 04/19/05 Project Design Specification 1 BioE 1160 Senior Design 2004-05 Bioengineering Department University of Pittsburgh, PA Standard Controlled Document Form Doc. No. DJKT-001 Rev. 2 Date: 04/19/05 Status: Draft Project Design Topic: Design of Distal Protection Filter for Carotid Artery Angioplasty and Stenting Problem Statement: Stroke is considered to be the third leading cause of death in the United States, accounting for 1.5 deaths reported per 1000 people. This disease is the most common and disabling cardiovascular problem that affects the elderly population. For the last few decades, the standard treatment for strokes has been carotid endarterectomy (CEA), which is based on the open surgical exposure of the carotid bifurcation to repair the stenosed artery. While this does improve the condition of the patient, there are complications and limitations due to wound problems. Recently, a new procedure has been developed for the treatment of the blockage in a blood vessel. This procedure, which is minimally-invasive, is called carotid artery stenting (CAS) which deploys a wire mesh to treat the occluded segment of the artery. However, the major concern about this procedure is its potential to produce emboli which could cause complications. In order to reduce this production of emboli, several devices have been recently developed as an adjunct to CAS. One of these devices that has received recent attention is the embolic protection filter (EPF), which allows distal perfusion. Although there have been some EPFs designed, none of these devices have the ability to completely prevent embolization into the carotid artery. The goal of this project then is to design a novel embolic protection filter that would be more efficient during CAS. Client Requirements: Machine should capture emboli as CAS is performed efficiently. Device should not be complicated—straightforward with some training required Device should minimize volume and maximize surface area. Device should be able to accommodate different types and sizes of emboli. Device should be made to fit in an artery without damaging the blood vessel. Device should contain pores in order for distal blood flow to occur during the procedure. Device should be light so that it can enter the blood vessel and be retrieved easily. Device should be easily accessible for any user of either gender. Design Requirements: 1) Operational requirements: Usage: The device must be accessible for medical use. The product should be able to be inserted in the body in a minimally-invasive method. This device should allow blood flow to occur during the CAS. The pore size of the filter should be efficient in capturing the emboli but allowing blood to flow through. This device also should maximize the efficiency in capturing the emboli produced by the CAS procedure. The device should be simple and uncomplicated to operate. Safety: The embolic protection filter needs to be packaged in a safe container. The filters also need to be sterilized in order to be used for treatment of cardiovascular problems. The filters also should be properly disposed of in a biohazard container. The EPF also should be safe to use during the Project Design Specification 2 BioE 1160 Senior Design 2004-05 Bioengineering Department University of Pittsburgh, PA Standard Controlled Document Form Doc. No. DJKT-001 Rev. 2 Date: 04/19/05 Status: Draft procedure. The EPF should not cause any damage to the arterial wall and it should not impede blood flow. The mesh wiring used for the filter should be safe inside the body. Operating Environment: The device will be subjected to laboratory, clinical, or animal facility conditions. It could be used for testing or for treatment of patients with arterial blockage. Durability: The filter needs to be able to resist blood flow as it is inserted into the patient. It must be able to endure the pressures involved with the blood flow. This device cannot collapse or break during CAS. This device needs to able to function properly until it is retrieved at the end of the procedure. 3) Aesthetics Device should be small and light. Color, texture, and form undetermined. Production Characteristics a) Material The materials used to produce the filter should be durable and reliable, but also cost efficient. b) Quantity One prototype c) Target Production Cost $100 Miscellaneous a) Standards and Specifications A certain range of filters for use needs to be determined. Also, approval from users will be needed. This filter must contribute to the CAS procedure in a way that is more convenient than the standard procedure (carotid endarterectomy) for treatment of strokes. b) Competition This filter must be more efficient than previous filters that have been designed. c) Maintenance, Shipping, Packing, and Materials Undetermined at this time Project Design Specification 3