Blood collection & I.. - Hepatitis Virus Research Group
advertisement

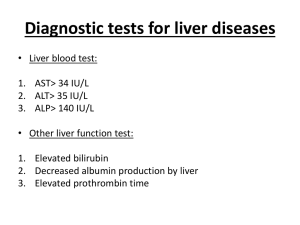
Author: Joe Grove – some of the collection protocol may be out of date. Protocol for collection of patient blood and isolation of IgG. Collection of patient blood: 3 consent forms (see attached) are required per patient, 1 for patient, 1 for doctor and 1 for researcher. Consult patient records and identify HCV +ve (HIV –ve) patients. Approach the consultant due to see the patient and ask them to gain consent and organise blood to be taken, aim to collect 20ml in red top vacutainers. Handling of HCV infected blood: All isolations to be carried out in tissue culture lamina No. 4. Protective gear (lab-coat, disposable plastic apron, gloves) must be worn while handling blood samples. Place all equipment/samples on a plastic tray (easier to clean in case of spillage). Only use disposable Pasteur pipettes, do NOT use needles or other sharp materials, or pipettes that are also used for routine cell culture work. Pipettes and all other plasticware (including vacutainers) contaminated with blood MUST be soaked in 10% Trigene solution for 24hrs prior to disposal in a sealable waste pot. Discard all material into yellow sharps bin (including closed vacutainers and paper towels); bin to be incinerated after use (do NOT leave in the hood for other people to use, even it is only half full!). Clean hood with 10% Trigene and 70% Ethanol after use. Immediately clean up spillages with paper towel and 10%Trigene. Do NOT pool any (clotted) blood from vacutainers into falcon tubes! Isolation of serum Collect in Red Top Vacutainers (+clot activator) Spin vacutainers 10 min at 3000 rpm (brake 4) at 4-6 C (in sealed buckets!) Carefully take off supernatant using a disposable Pasteur pipette and transfer to Falcon tubes, make sure not to disturb the red blood cells (if you accidentally mix serum/plasma and red blood cells, re-spin supernatant for 5 min and transfer to fresh falcon tube) Heat inactivate sera by incubation at 56C for 1 hour. Remove other objects and glass thermometer from water bath prior to doing so and clean water bath with 70% ethanol after use. Separate sera in to 2.5 ml aliquots and store in locked -80. – tubes labelled with “biohazard” sticker and stored in re-sealable plastic bag. IgG isolation from patient sera. Using designated HCV+ve or HCV-ve 4ml protein G column stored in cold room in IgG isolation box. 1. Filter samples, to do this; dilute 1/4 in PBS, spin @ 2000rpm for 10mins and 0.2uM syringe filter in to fresh tube. 2. Wash column with 10 mls sterile PBS 3. Pass sample through column to capture IgG. 4. Wash column with 10mls PBS. 5. Elute IgG with 12mls 0.1M Glycine (pH2.7) discarding the first 2ml, collecting the remainder. 6. pH test the elute and neutralise with 1M tris pH 9.0 (~650ul/10ml) 7. Wash column with 10mls PBS. 8. Pass 10mls PBS 0.1% NaN3 through column before storage. 9. Dialyse IgG over night against PBS. 10. 0.2uM filter sterilise IgG. 11. Quantify protein concentration of a 1/100 dilution of IgG using a spectrophotometer @ 280nm, use following formula. (X/1.35) X 100 = [IgG] (mg/ml) Any equipment that has come into contact with patient sera must be soaked in 10% Trigene for 24hrs and then placed into a yellow sharps bin which must be disposed of immediately, as above. CONSENT FORM Study Title: The Immune System in Chronic Liver Diseases 1. I confirm that I have read and understand the information sheet dated 20th August 2007 for the above study. 2. I understand that my blood donation for this research is voluntary and that I am free to withdraw participation at any time without my medical care or legal rights being affected. 3. I understand that isolated blood will be stored for a limited time until the experiments are competed. 4. I agree for 25ml of blood (about 2 full table spoons) to be taken for this study. 5. I agree to take part in the above study. Name of patient Date Signature Name of person taking Consent (if different from Principal Investigator) Date Signature Principal Investigator Date Signature 1 copy for patient, 1 for researcher, 1 to be kept with hospital notes Information sheet 3 (for patients with chronic liver disease) 20 August 07 The Immune System in Chronic Liver Diseases Please keep this information sheet for future reference 1. Introduction to the Research. This study is being organised and funded by the Liver Unit in Birmingham. We are interested in how your white blood cells which as part of the immune system have evolved to fight infections might also be involved in the development of liver diseases. 2. What is the research study about? We believe that the immune system plays an important role in the normal liver by fighting bacteria and viruses to prevent them from infecting the liver. However, under some circumstances white blood cells can be misdirected to damage normal tissues leading to chronic liver damage. In our studies we will isolate white cells from blood taken form your vein. We can then grow these cells in the laboratory and study how they might lead to liver damage. For some experiments it is necessary to store the isolated cells and/or small pieces of tissue for subsequent experiments. 3. Why have I been chosen? Because you are chronically infected with Hepatitis C virus. 4. What will I have to do? If you agree to take part in our research study we would like to take a blood sample from you (about 2 tablespoons of blood, 25 ml). This blood sample is taken in addition to the routine samples taken but it can be done when you are having blood removed for your routine tests. The removal of this small amount of blood will not in any way effect your condition as your body can rapidly replace the cells removed. 5. What are the benefits? There is no direct benefit to you from taking part although the information we gain may help us to understand liver diseases and develop new treatments in the future. 6. What are the risks? There are no additional risks for you. 7. What are the alternatives? This study does not involve any change in treatment and you are free to not take part in it. 8. What if I do not want to take part? Your participation in the study is entirely voluntary and will not affect your present or subsequent care in any way. If you decline to be involved or subsequently withdraw from the study this will also have no effect on your present or future treatment. 9. What happens to the information? Your participation in this study will be confidential. No personal information which could identify you will leave the hospital. Any samples that are stored will not be labeled with you personal information. 10. Are there any restrictions on what I might eat or do? There are no restrictions on what you can eat or do apart from those that relate to your routine treatment. 11. Confidentiality agreement. Your participation in this study will be confidential. identify you will leave the hospital. No information which could 12. Who else is taking part? Other patients who are chronically infected with Hepatitis C. 13. What if something goes wrong? The study does not involve any change in your treatment so there are no risks for you. 14. What happens at the end of the research study? The results of this research will be kept and published so that other research groups can benefit from the results gained. Cells and tissue samples that are not longer needed will be destroyed. 15. Is my doctor being paid for including me in the study? No, neither your doctor nor the hospital receives any money. 16. What if I have more questions or do not understand something? If you have any questions please ask your doctor, or contact the Liver Research Laboratories using the number below. Should you wish to see the overall results of the study, or the publication, which may result, please ask your study doctor. 17. What happens now if I decide to take part? If you decide to take part, we will ask you to take more blood than we need to treat you (about 2 tablespoons full) and will use the blood for our research. 18. What happens if I change my mind during the research study? If you change your mind at any time, do let us know and we will destroy any samples we have already taken. 19. This study has been reviewed by the South Birmingham Local Research Ethics Committee. 20. Contact name and number If you need any further information this can be obtained from Professor David Adams or Dr. Mathis Heydtmann who can be contacted via the hospital switchboard (0121-472-1311).