this poster
advertisement
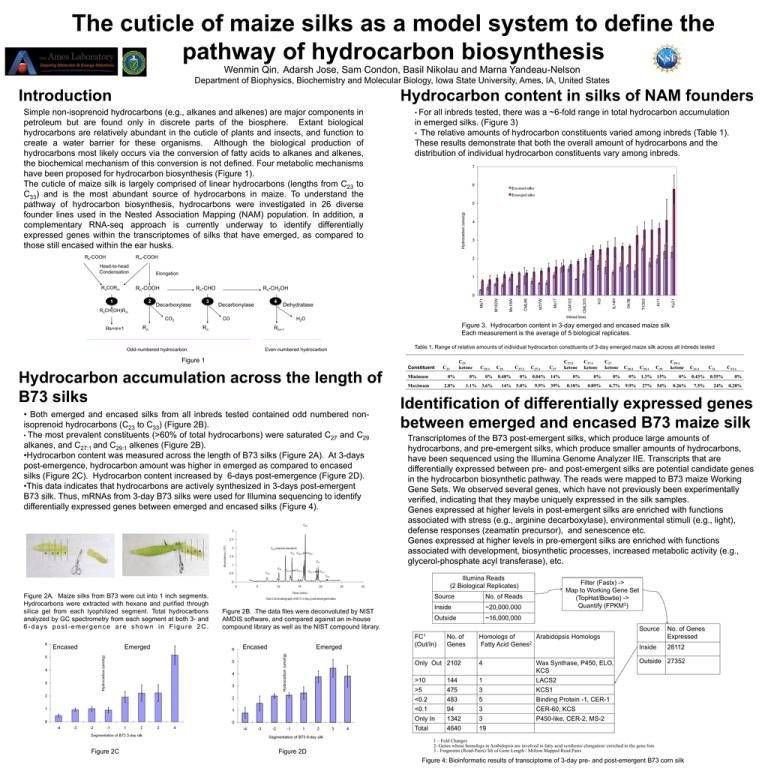
The cuticle of maize silks as a model system to define the pathway of hydrocarbon biosynthesis Wenmin Qin,Adarsh Jose, Sam Condon, Basil Nikolau and Marna Yandeau-Nelson Department of Biophysics, Biochemistry and Molecular Biology, Iowa State University, Ames, IA, United States Introduction Hydrocarbon content in silks of NAM founders Simple non-isoprenoid hydrocarbons (e.g., alkanes and alkenes) are major components in petroleum but are found only in discrete parts of the biosphere. Extant biological hydrocarbons are relatively abundant in the cuticle of plants and insects, and function to create a water barrier for these organisms. Although the biological production of hydrocarbons most likely occurs via the conversion of fatty acids to alkanes and alkenes, the biochemical mechanism of this conversion is not defined. Four metabolic mechanisms have been proposed for hydrocarbon biosynthesis (Figure 1). The cuticle of maize silk is largely comprised of linear hydrocarbons (lengths from C23 to C33) and is the most abundant source of hydrocarbons in maize. To understand the pathway of hydrocarbon biosynthesis, hydrocarbons were investigated in 26 diverse founder lines used in the Nested Association Mapping (NAM) population. In addition, a complementary RNA-seq approach is currently underway to identify differentially expressed genes within the transcriptomes of silks that have emerged, as compared to those still encased within the ear husks. Rs-COOH • For all inbreds tested, there was a ~6-fold range in total hydrocarbon accumulation in emerged silks. (Figure 3) • The relative amounts of hydrocarbon constituents varied among inbreds (Table 1). These results demonstrate that both the overall amount of hydrocarbons and the distribution of individual hydrocarbon constituents vary among inbreds. Rm-COOH Head-to-head Condensation RsCORm Elongation Rn-COOH 1 2 Rn-CHO 3 Decarboxylase Rn-CH2OH 4 Decarbonylase Dehydratase RsCH(OH)Rm CO2 Rs+m+1 CO Rn H2O Rn Odd-numbered hydrocarbon Figure 3. Hydrocarbon content in 3-day emerged and encased maize silk Each measurement is the average of 5 biological replicates. Rn+1 Even-numbered hydrocarbon Table 1. Range of relative amounts of individual hydrocarbon constituents of 3-day emerged maize silk across all inbreds tested Figure 1 Constituent Hydrocarbon accumulation across the length of B73 silks • Both emerged and encased silks from all inbreds tested contained odd numbered nonisoprenoid hydrocarbons (C23 to C33) (Figure 2B). • The most prevalent constituents (>60% of total hydrocarbons) were saturated C27 and C29 alkanes, and C27:1 and C29:1 alkenes (Figure 2B). •Hydrocarbon content was measured across the length of B73 silks (Figure 2A). At 3-days post-emergence, hydrocarbon amount was higher in emerged as compared to encased silks (Figure 2C). Hydrocarbon content increased by 6-days post-emergence (Figure 2D). •This data indicates that hydrocarbons are actively synthesized in 3-days post-emergent B73 silk. Thus, mRNAs from 3-day B73 silks were used for Illumina sequencing to identify differentially expressed genes between emerged and encased silks (Figure 4). C29 -4 -3 -2 1 -1 2 3 C26 (internal standard) 4 C27 C29:2 and C29:1 C31 C25 C23 C27:2 and C27:1 Encased Emerged Figure 2C Emerged Figure 2D C25:1 C25 C27:2 C27:1 C27 C27:1 ketone C27 ketone C29:2 C29:1 C29 C29:1 ketone C31:1 C31 C33:1 0% 0% 0% 0.48% 0% 0.04% 14% 0% 0% 0% 0% 1.3% 15% 0% 0.43% 0.55% 0% Maximum 2.8% 1.1% 3.6% 14% 5.0% 9.9% 39% 0.18% 0.89% 6.7% 9.9% 27% 54% 0.26% 7.5% 24% 0.28% Identification of differentially expressed genes between emerged and encased B73 maize silk Transcriptomes of the B73 post-emergent silks, which produce large amounts of hydrocarbons, and pre-emergent silks, which produce smaller amounts of hydrocarbons, have been sequenced using the Illumina Genome Analyzer IIE. Transcripts that are differentially expressed between pre- and post-emergent silks are potential candidate genes in the hydrocarbon biosynthetic pathway. The reads were mapped to B73 maize Working Gene Sets. We observed several genes, which have not previously been experimentally verified, indicating that they maybe uniquely expressed in the silk samples. Genes expressed at higher levels in post-emergent silks are enriched with functions associated with stress (e.g., arginine decarboxylase), environmental stimuli (e.g., light), defense responses (zeamatin precursor), and senescence etc. Genes expressed at higher levels in pre-emergent silks are enriched with functions associated with development, biosynthetic processes, increased metabolic activity (e.g., glycerol-phosphate acyl transferase), etc. C31:2 and C31:1 Figure 2B. The data files were deconvoluted by NIST AMDIS software, and compared against an in-house compound library as well as the NIST compound library. Encased C23 C27:2 ketone Minimum C33 Figure 2A. Maize silks from B73 were cut into 1 inch segments. Hydrocarbons were extracted with hexane and purified through silica gel from each lyophilized segment. Total hydrocarbons analyzed by GC spectrometry from each segment at both 3- and 6-days post-emergence are shown in Figure 2C. C23 ketone Illumina Reads (2 Biological Replicates) Source No. of Reads Inside ~20,000,000 Outside ~16,000,000 Filter (Fastx) -> Map to Working Gene Set (TopHat/Bowtie) -> Quantify (FPKM3) Source Homologs of Arabidopsis Homologs Fatty Acid Genes2 No. of Genes Expressed Inside 28112 Only Out 2102 4 Outside 27352 >10 >5 <0.2 <0.1 Only In Total 1 3 5 3 3 19 FC1 (Out/In) No. of Genes 144 475 483 94 1342 4640 Wax Synthase, P450, ELO, KCS LACS2 KCS1 Binding Protein -1, CER-1 CER-60, KCS P450-like, CER-2, MS-2 1 – Fold Changes 2- Genes whose homologs in Arabidopsis are involved in fatty acid synthesis/ elongation/ enriched in the gene lists 3 - Fragments (Read-Pairs) /kb of Gene Length / Million Mapped Read Pairs Figure 4: Bioinformatic results of transciptome of 3-day pre- and post-emergent B73 corn silk