Feb2013_TulsaCC_The-rAmyProject
advertisement

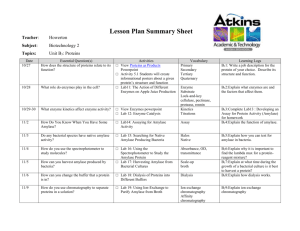
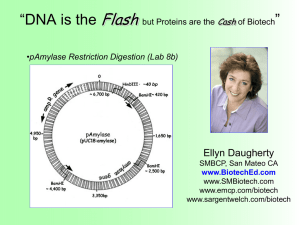
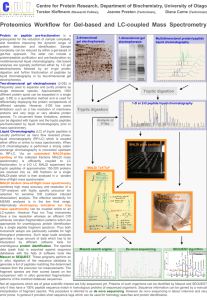
The rAmylase Project Faster, Better Biotech Ellyn Daugherty SMBCP, San Mateo CA www.BiotechEd.com www.SMBiotech.com www.emcp.com/biotech www.sargentwelch.com/biotech Tulsa Community College February 22-23, 2013 The rAmylase Project (Faster, Better Biotech) Friday 2/22/12 5-8 pm 5:00 pm Amylase collection (before you eat – spit into a tube) 5:15 pm Welcomes/Light dinner/Intro to The rAmylase Project 5:45 pm Set up Lab 6c Amylase Assay 6:45 pm Part I - Amylase ELISA Kit (Prepare/plate samples) 7:45 pm Plan for Saturday (9 am sharp!) Sat 2/23/12 9-4 pm 9:00 am 10:30 am 11:30 am 12:30 pm 1:15 pm 3:45 pm Part II - Amylase ELISA Kit (add Ab, colorimetric visualization) Lab 8b pAmylase Restriction Digestion (kit) Prepare samples & set up for gel electrophoresis Run gels and visualize (1XTAE gels vs 1X LB gels) Lab 5f Amylase PAGE (set up gel, load, run gels during lunch) working Lunch (results & Intro to Column Chromatography) Lab 9c Amylase Chromatography (kit) "What now?" In Biotech, companies find a product of interest (amylase) and make sure to have assays to show it’s presence, activity, & concentration Amylase is an enzyme that catalyzes starch digestion. It is used commercially in several ways including: 1) Remove starch in products 2) Produce sugar from starch 3) Processing of cellulostic biofuel The rAmylase Project is the focus of the 2nd semester of the SMBCP • Learn how to assay for a protein of commercial interest (amylase) • Transform cells to produce that protein (using pAmylase and C2523 cells) • Scale-up cells to volumes where amylase can be purified and assayed Absorbance (a.u) Ion Exchange Chromatography 0.35 0.3 0.25 0.2 0.15 0.1 0.05 0 -0.05 0 5 Fractions (#) 10 The rAmylase Project Model of rDNA/protein Business < Chapters 1-5 Basic SLOP < Lab 5f Amylase PAGE (revised) < Lab 6e Amylase Producing Bacteria < Lab 6c Amylase Activity Assay (revised) < Lab 6d Amylase ELISA and W Blot (new) < Labs 7a/7b/7f/7g Amy Spectrophotometry < Lab 8b Restriction Digestion of pAmylase < Lab 8b DNA Gel Electrophoresis (revised) < Lab 8c pAmy Transformation of E. coli < Lab 9c/9d Amy Ion-Ex Chromatography < Lab 8g/4h Genomic & plasmid DNA Isolation < Lab 13i Amylase Gene PCR (new) The San Mateo Biotechnology Career Pathway ELISAs taught in Biotech 2 (Ch 6) and Biotech 4 (Ch 14) Lab 6c (modified) Amylase Activity Assay Basic Procedure: • 2% (modified) starch is added to wells. • Amylase known or unknown is added to well. 3 min incubation. • Add Iodine to determine starch remaining. Introduction to ELISA ELISA – short for enzyme-linked immunosorbant assay An ELISA uses antibodies with conjugated enzymes to specifically identify and measure the amount (concentration) of a protein in a solution ELISA •A specific antibody (Ab) recognizes a specific protein (Ag). •An enzyme bound to the Ab causes a color change in a substrate (so you can “see” the Ab is there). •The more color in a well, the more Ab-enz complex is bound to target protein = higher target protein concentration. Di Lab 6d Amylase Elisa Kit Expected Results <<<<< The Standards should look like this … …and the unknowns should look something like this >>> Automated ELISA Plate Reader Lab 8b Restriction Digestion of pAmylase (kit) Basic Procedure: •Restriction enzyme is added to pAmylase plasmid tubes. •Mixture is incubated for 15-30 min at 37°C. •Samples run on agarose gel and visualized on UV imager. •Band pattern can confirm size and sequence of plasmid. Fast Restriction Digestions (HF)and Fast Agarose Gels (LB) NEB High Fidelity (HF) Restriction Enzymes http://www.neb.com These engineered enzymes have the same specificity as their established counterpart with the benefit of reduced star activity and: •Fast digestion times (5-15 min) •Work in same buffer (Buffer 4) BamHI-HF™ HindIII-HF™ Lithium Borate(LB) Buffers http://www.fasterbettermedia.com Lithium borate conducts electricity better than TRIS so you can turn up the voltage and current on agarose gels : • Fast gel run times (8-15 min) • Same protocol as TAE gels just substitute 1X LB buffer for 1X TAE • Purchase as 10X or 20X concentrate Lab 5f (Modified) Amylase on vertical SDS-PAGE Basic Procedure: • Prepare diluted samples of known amylase concentration with SPLD/BME. • Load and run on NuSep gels at 200-250V ! for 30 min. • View immediately on UV imaging system and/or stain with Coomassie blue (long or short method). Visualizing Protein Samples on Gels NuSep gel – UV photo: • Rinse in dH2O, then to viewing • 2 min UV exposure • Amylase #1-4, Rennin #5-8 NuSep gel – NuBlue stain photo: • 15- 30 min stain, no de-stain • May reuse • Rennin #3-6, Amylase #7-10 Using Chromatography to Study and Separate Molecules Paper chromatography. Molecules separate as they move up the paper. The distance that the molecules travel depends on their size and solubility in the solvent. Thin-layer chromatography. Molecules separate as they move through the silica gel. Thin-layer chromatography is used to separate small molecules, such as amino acids. Column Chromatograaphy Open or Gravity Column Ion-Exchange Chromatography Ion Exchange Resin. Resins are manufactured with ions attached. The ions present a certain degree of positive or negative charge, depending on the buffer pH. Column Chromatography in Biomanufacturing Fast-Performance Liquid Chromatography (FPLC) Pumps push the buffer or sample through tubing, into and through the column. As fractions come off the column, they are run through a spectrophotometer that determines the protein concentration of the sample. Column Chromatography in Biomanufacturing Fast-Performance Liquid Chromatography (FPLC) High-Performance Liquid Chromatography (HPLC) Greatly improved ability to separate, purify, identify, and qualify samples. Get more help… SMBCP Link IRC Link SW Lists Current Events LN’s workshops New Things/Upd ates All of LN’s PPTs