Affinity Chromatography
advertisement

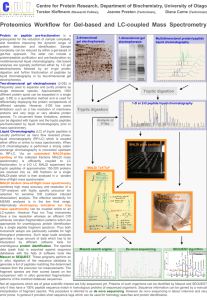
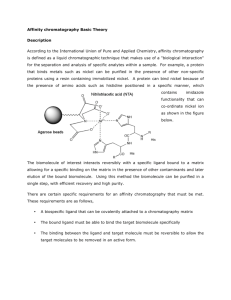
Chromatography Desalting and Affinity Chromatography • Technique to separate components of a mixture by passing them through a matrix. – A solvent is used for carrying sample mixture through the matrix – Separation occurs because each compound in a mixture interacts differently with the matrix. Gel-Filtration • Ammonium sulfate interferes with affinity chromatographic step. • We have to desalt our extract by using either dialysis or gel filtration. • Gel filtration chromatography is a separation based on size. It is also called molecular exclusion or gel permeation chromatography. Gel-Filtration • Separation mechanism is not based on adsorption. • It is independent of the eluent system • Has high recoveries both on basis of mass and biological activity. • Since it is iso-cratic, samples are diluted. Gel- Filtration • The volume between the beads is called as void volume – accessible to all molecules. • The volume in the pore is the pore volume • The non-porous part of the beads is the backbone volume not accessible to samples. • Partition occurs between the molecules that have access to the void volume and molecules that have access to void and pore volume. Gel-Filtration • Three steps – Equilibration – Sample loading – Elution Picture from Amersham website • Pores in the gel Desalting • Group Separation Mode: Used in separating small molecules from large molecules in a mixture – Macromolecules have access only to the void volume and therefore grouped and eluted together. – Small molecules (neutral salts, buffer salts, low mw additives) are separated as a single unit – as late as possible. They have access to pore volume. • The large difference in the elution volumes allow sample volumes up to 30% of the column volume. • High flow rates can be applied and broad or narrow columns can be used. Sephadex • Sephadex -50 is the matrix which exploits both physical and chemical properties of the mixture. • These highly specialized gel filtration and chromatographic media are composed of macroscopic beads synthetically derived from the polysaccharide, dextran(polymer of glucose). • The organic chains are cross-linked to give a three dimensional network having functional ionic groups attached by ether linkages to glucose units of the polysaccharide chains. • Available forms include anion and cation exchangers, as well as gel-filtration resins, with varying degrees of porosity; bead sizes fall in discrete ranges between 20 and 300µ. http://web.siumed.edu/~bbartholomew/images/protein_methods/sephadex.gif Affintiy Chromatography • Affinity chromatography (AC) is a technique enabling purification of a biomolecule with respect to biological function or individual chemical structure. • The substance to be purified is specifically and reversibly adsorbed to a ligand (binding substance), immobilized by a covalent bond to a chromatographic bed material (matrix). • Samples are applied under favourable conditions for their specific binding to the ligand. • Substances of interest are consequently bound to the ligand while unbound substances are washed away. • Recovery of molecules of interest can be achieved by changing experimental conditions to favour desorption. AC media are commonly used for applications such as purification of fusion proteins, mono- and polyclonal antibodies, and glycoproteins. Affinity Chromatography (AC) • AC involves an inert matrix coupled with a affinity ligand specific for a binding site on the target molecule. • Under suitable binding conditions this affinity matrix will bind molecules according to its specificity only. • All other sample components will pass through the medium unadsorbed. Affinity Chromatography • After washing, condition are changed to disassociate or displace the molecules from the ligand. • simple "on-off" mode of chromatography is applied by switching abruptly from full binding to complete release conditions. • Thus AC fishes out the target molecule by way of highly specific binding and release, rather than removing contaminants by fine-tuned isocratic or gradient elution techniques. Affinity Chromatography • Two important things to consider; – Finding a ligand specific enough to allow step elution. – Finding conditions for safe binding and release within the stability window of the target molecule and the ligand. Affi-Gel Blue • Agrose gel covalently attached Cibacron Blue F3GA dye. • ≥1.9mg dye per mL of gel • We are using 50-100 mesh (150-300 µm) • Separates nucleotide dependent enzymes (dinucleotide fold biospecifically bind to the dye) • Enzyme can be eluted from the dye with a specific nucleotide co-factor Sigma-Aldrich site