A seminar on Validation of Dry Powder Mixers

A seminar on
Validation of Dry Powder
Mixers
By
WWW.PARASSHAH.WEEBLY.COM
M.Pharm. (Sem - III)
Department of Quality Assurance
Maliba Pharmacy College.
1
Contents
Introduction variables
Powder mixers validation
Revalidation
Latest advancements in the blend analysis
References
2
Introduction
Validation of Dry Powder Mixers
It is defined as documented act which provide the high degree of the assurance that
Powder Mixer equipment actually leads to the desired mixing or blending.
Why it is essential
The mixing of the API and excipients is the critical step in the solid dosage form preparations that affect the content uniformity at great extent
3
Types of the powder blenders
V cone blenders
Double cone blenders
Drum mixer
Ribbon blenders
Conical screw mixer
Tumble blender
4
Variable and monitoring
Variable
RPM
Mixing time
Mixing load
Monitoring
Blend uniformity
5
Worst case
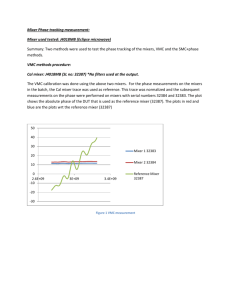
Evaluation of worst case: Worst case include, maximum and minimum load, maximum and minimum rpm, maximum and minimum time.
6
URS for the powder mixers
Operating criteria must be adequate
Spares should be available
Easy maintenance
Equipment should not disseminate dust
Low cost
Non reactive surface
Capacity
Mixing speed
7
Installation qualification
Details of the Equipment
Equipment name, made by & model No. shall be noted down.
Location for the installation equipment shall be checked.
Utilities required shall be listed down.
Any deviation observed while following above procedure should be informed for corrective action.
Installation Procedure:
After checking all the specifications as mentioned in the selection criteria, service engineer shall commission the equipment.
Authorized validation team shall carry out installation checks.
8
4.
5.
6.
7.
2.
3.
Sr no.
1.
Description
Equipment type
Specifications
Capacity (L)
Dimensions H
L
W
Surface finish
Driving motor Made by
RPM
Voltage
Phase
Gear box Made by
Type
Control panel & buttons
Method of evaluation
Check visually
Observation
Measure tape
Check visually
Check visually
Check visually
Check visually
9
Operational qualification
After completions of successful installation qualification initiate the actual operation of to ensure that machine is operating within specification.
Check the operation qualification parameters against their specifications.
Document the deviation details
The Quality head and the department head shall decide whether deviation is acceptable or not.
10
Sr no.
Description
1.
On/off switch
Specifications Method of evaluation Observation
2.
3.
RPM
Gross capacity
Lift the switch to ON position & ensure that power supply gets ON & drum/cone starts rotating.
Lower the switch to OFF position & ensure that power supply gets OFF
Measure the actual RPM using stop-watch
Fill the drum/cone with potable water using measuring cylinder & record
11
Performance qualification
Load the materials to be mixed in the mixer
Start the mixer and rotate it for the time as mentioned in the BMR.
After completion of mixing switch OFF the mixer and separate out the drum.
Collect the sample as per sampling procedure.
Send the samples to Quality control dept. for content uniformity, bulk density and sieve analysis.
12
Sampling
Drum mixer Double cone blender V cone blender
Top
Middle
Bottom 13
Content uniformity
Sampling location
Top
Middle
Bottom
In process parameter
Content uniformity
Sieve Analysis
Sieve analysis
Retained on
20#
Retained on the 40#
Retained on
60#
Passed through 60#
Top Middle
Result
Bottom
%RSD
%RSD
14
Density
Density
Bulk
Tapped
Top Middle Bottom %RSD
15
Revalidation Criteria
Location of the equipment is changed.
There is change of spare/ parts that have a direct effect on the performance of the equipment
At normal revalidation schedule.
16
Latest advancements in the Blend analysis
1.
2.
3.
NIR spectroscopy
Raman spectroscopy
Microscopic FTIR mapping
17
Online Monitoring with NIR probe
18
Online Monitoring with Raman probe
19
References
http://www.validationonline.net/Mixer.html
www.askaboutvalidation.com
Pharmaceutical Master Validation Plan by Sayed
Imtiyaz Haider published by st. Luice press page no 125.
A Report of the Product Quality Research
Institute Workshop on Blend Uniformity by Jozef
H.Timmermans
20
Thank you
21