Intermolecular Forces

Intermolecular Forces
Forces between molecules
Intermolecular Forces (IMFs)
Different molecules have different forces that act between them.
These forces attracting the separate molecules together control many physical properties
Boiling Point
Melting Point
Solubility
Viscosity
Surface Tension
Three Main IMFs
Dipole-dipole forces
Hydrogen bonding
London dispersion forces (LDFs)
Dipole-Dipole Forces
Electronegativity – the ability of an atom in a compound to attract electrons to itself.
Fluorine has the highest electronegativity
The electrons in a compound spend more time around the most electronegative atoms than the other atoms.
Electronegativity
If the electrons are spending more time around the one particular atom, how will that atom be different from the other atoms?
It will have a more negative charge than the other atoms in the compound.
Does not have a full negative charge but a partial negative charge ( δ , lower case delta)
Hydrofluoric Acid (HF)
The red end (fluorine) has a partial negative charge.
Polar Molecules
One end of the molecule is positive while the other end of the molecule is negative.
This difference in charge is called a
“dipole”
What effect does this have?
How does this change the way two molecules interact?
The positive end of the molecule is attracted to the negative end of a different molecule.
How to spot dipole-dipole forces?
Look for molecular shapes that have uneven placements of atoms.
Bent
Trigonal pyramidal
Anything that has more than one type of atom around the outside
Is Carbon Dioxide Polar?
Is carbon tetrafluoride polar?
Is Water Polar?
Why is Polarity Important
Things that are polar or have charges dissolve in things that are polar.
Things that are nonpolar dissolve in things that are nonpolar.
“Like dissolves Like”
Opposing types do not dissolve in each other.
A Bio Reminder
Hydrophilic – “water loving” – polar
Hydrophobic – “water fearing” - nonpolar
Dissolution Process
How are strong ionic bonds broken in water?
Dissolving_NaCl-Electrolyte.exe
The polar nature of water creates attractions between the water and ionic compound.
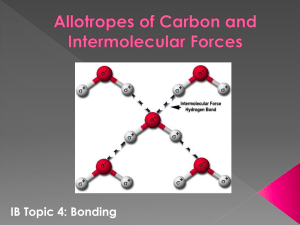
Hydrogen Bonding
Special case of dipole-dipole forces.
The difference in electronegativity between some atoms and hydrogen is so strong that it creates a very strong dipole
What elements can do this?
Which elements have the strongest electronegativity?
Anything with an N-H bond, O-H bond, or
F-H bond will have hydrogen bonding.
Hydrogen Bonding is Very Important
It is the reason why ice floats.
Hydrogen Bonding is Very
Important
DNA Base Pairs
London Dispersion Forces (LDFs)
Often called “induced dipoles”
A momentary change in where the electrons are in one molecule, “induces” a dipole in another molecule.
LDFs
The more electrons you have in a molecule, the more likely you are to have momentary imbalances in charges.
The more electrons in an atom, the stronger the London Dispersion Forces.
Any molecule can have London Dispersion
Forces.
LDFs
This explains why the boiling point goes up as you move down a column.
Hydrogen telluride has more electrons than hydrogen sulfide
Hydrogen telluride has stronger LDFs
Hydrogen telluride has a higher boiling point
Crude Oil
Crude oil is refined based on differences in LDFs.
Longer carbon chains have higher boiling points
Have larger number of electrons
IMF Comparison
LDFs are the weakest
Dipole-dipole are in the middle
Hydrogen bonding is the strongest.
Practice Problems
List all of the intermolecular forces acting on two phosphorus trichloride molecules
Figure out the formula
Draw a Lewis structure
Figure out the molecular geometry
Check to see what IMFs it has.
Practice Problem
Explain why ammonia (-33.4
º C)has a higher boiling point than phosphine, PH
3
(-87.8
º C).
Justify your answer.
Figure out ammonia’s formula
Draw Lewis structures for both compounds
Figure out the molecular geometry for both compounds
Figure out what IMFs each compound has.
Compare the two compounds.
Practice Problems
Explain why ammonia (-33.4
º C)has a higher boiling point than phosphine, PH
3
(-87.8
º C).
Justify your answer.
Practice Problem
Hexane (C
6
H
14
) is a liquid at room temperature. Its Lewis structure has each carbon connected to another in a long chain. Will sodium chloride dissolve in hexane? Justify your answer.