Intermolecular Forces - slider-dpchemistry-11
advertisement

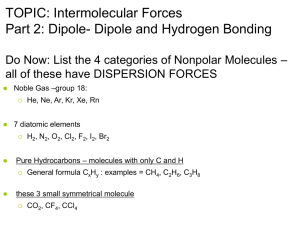
Intermolecular Forces 11 DP Chemistry London Dispersion Forces The temporary separations of charge that lead to the London force attractions are what attract one nonpolar molecule to its neighbors. This is due to the constant movements of electrons Fritz London 1900-1954 Dispersion forces increase with the size of the molecules due to the presence of more electrons. They are also known as Van der Waal forces. London Forces in Hydrocarbons Dipole-Dipole Attractions Attraction between oppositely charged regions of neighboring covalent molecules. The negative and positive regions of these molecules represent regions of higher and lower electronegativity, respectively. The water dipole The ammonia dipole Hydrogen Bonding Bonding between hydrogen and more electronegative neighboring atoms fluorine, oxygen and nitrogen Hydrogen bonding in Kevlar, a strong polymer used in bulletproof vests is represented by the dashed lines. Hydrogen Bonding in Water Hydrogen Bonding between Ammonia and Water Evidence for strength of H-bonding HF and HCl are similar molecules and both have polar bonds However, their boiling points are very different indicating much greater IM forces between HF. Boiling points (0C) HCl HF -85 20 Notice this trend is the same for all hydrides containing N, O, F. Another Example Any molecule which has a hydrogen atom attached directly to an oxygen or a nitrogen is capable of hydrogen bonding. Such molecules will always have higher boiling points than similarly sized molecules which don't have an -O-H or an -N-H group Ethanol, CH3CH2-O-H, and methoxymethane, CH3-O-CH3, both have the same molecular formula, C2H6O. Ethanol Methoxymethane 78.5°C -24.8°C Referring to the structure, explain why the bp difference ANSWER: Van der Waals forces are similar - they have the same number of electrons and a similar length. However, ethanol has a hydrogen atom attached directly to an oxygen with two lone pairs as in a water molecule. Hydrogen bonding can occur between ethanol molecules, although not as effectively as in water. The hydrogen bonding is limited by the fact that there is only one hydrogen in each ethanol molecule with sufficient + charge. In methoxymethane, the lone pairs on the oxygen are still there, but the hydrogens aren't sufficiently + for hydrogen bonds to form. Except in some rather unusual cases, the hydrogen atom has to be attached directly to the very electronegative element for hydrogen bonding to occur. Relative magnitudes of forces The types of bonding forces vary in their strength as measured by average bond energy. Strongest Covalent bonds (400 kcal) Hydrogen bonding (12-16 kcal ) Dipole-dipole interactions (2-0.5 kcal) Weakest London forces (less than 1 kcal) Exercises 1. H20 and H2S have the same molecular shape. However, they have very different boiling points (100 and -600C respectively) Draw these molecules and explain the difference in b.p. values. 2. What would you predict about the relative b.p. values of NH3 and PH3? 3. Compare the relative strengths of IM forces for the three compounds below (propane, ethanal and ethanol).