Unit 7 Revision
advertisement

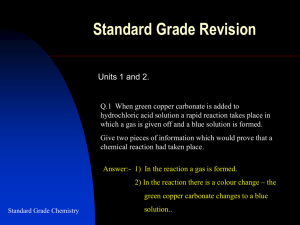
Standard Grade Revision Unit 7 Q1. The word box contains the names of some elements. sulphur mercury phosphorus sodium carbon helium (a) Identify the non-metal element which is a conductor. (b) Identify the two other elements that are conductors. (a) Carbon. (b) Mercury and sodium. Q2. The word box contains the names of some compounds . solid sodium chloride molten candle wax (a hydrocarbon) molten lead bromide solid sucrose ( a carbohydrate) liquid carbon tetrachloride copper nitrate solution. (a) Identify the two substances which are conductors. (b) Identify the charged particle which carries the current in both of the conductors. (a) Molten lead bromide and copper nitrate solution. Standard Grade Chemistry (b) Ions. Unit 7 Revision Q3. Substances can be classified as conductors or non-conductors and also as solids or liquids. Substance State Conductor or non-conductor A solid non-conductor B liquid non-conductor C solid conductor D liquid conductor (a) Which two substances could be sodium chloride? (a) A and D (b) Which substance could not be a compound? (b) C Q4. Identify the two statements which refer to an atom of fluorine. You may wish to use the data booklet to help you. Standard Grade Chemistry A It has a stable electron arrangement B It will form an ion by losing one electron. C It will form an ion with a single negative charge. D It has two more electrons than an oxygen atom. E It has the same number of electrons as a chlorine atom F It has the same number of outer electrons as an iodine atom C and F Unit 7 Revision Q5. Gemma was investigating electrical conductivity. She set up four experiments. Identify the two experiments in which the bulb will light. C and D Standard Grade Chemistry Unit 7 Revision. Conducts as Q6. Substance a solid Melting point a liquid / oC A no yes 801 B no no 113 C yes yes 63 D no no 1700 E yes yes 98 F no no 44 (a) Which substance could be sodium chloride? (a) A (b) Which two substances are made up of molecules? (b) B and F (c) Which substance has a covalent network structure? (c) D (d) Which two substances are metals? (d) C and E Q7. Titanium(IV) chloride is a liquid at room temperature. What type of bonding does this suggest is present in Standard Grade Chemistry titanium(IV) chloride? covalent Unit 7 Revision. Number of Q8. Particle protons neutrons electrons A 12 13 12 B 8 10 10 C 12 12 10 D 11 13 10 E 8 10 8 (a) Identify the particle which is a negative ion. (a) B (b) Identify the particle which is an ion with a charge of 1+. (b) D (c) Identify the particle is an atom of a non-metal element. (c) E (d) Identify the two particles which are isotopes. (d) A and C Q9. The box below shows a number of ions. F- Mg 2+ Cl - O 2- Al 3+ S 2- (a) Which two ions have the same electron arrangement as an argon atom? Standard Grade Chemistry (b) Which ion has 10 electrons and 12 protons? (a) Cl- and S2- (c) Write the ionic formula of the compound aluminium oxide. (b) Mg2+ (c) (Al3+)2(O2-) 3 Unit 7 Revision. Q10. Electrolysis of a solution of copper(II) chloride produced copper at the negative electrode and chlorine at the positive electrode. (a) What is meant by the term ‘electrolysis’? (b) Write an ion-electron equation for (i) the formation of copper at the negative electrode. (ii) the formation of chlorine at the positive electrode. (a) Electrolysis occurs when an electric current is used to break down an ionic compound in solution (or in a melt). (b) (i) Cu 2+(aq) + 2e- Cu(s) (ii) 2Cl -(aq) Cl2(g) + 2e Q11. Standard Grade Chemistry Write the formulae for the following compounds:(a) Sodium carbonate. (a) Na2CO3 (b) Lead(II) nitrate (b) Pb(NO3) 2 (c) Iron(III) hydroxide. (c) Fe(OH) 3 (d) Barium sulphate (d) BaSO4 Unit 7 Revision. Q12. The structure of a boron atom can be represented in a simple diagram. (a) Draw a similar diagram to show the structure of a fluorine atom. You may wish to use page 1 of the data booklet. . .. . . .. . . . Standard Grade Chemistry Fluorine atom Fluoride ion (b) Draw a similar diagram to show the structure of a fluoride ion. Unit 7 Revision. Q13. Martin set up the following experiment. (a) What type of experiment did Martin carry out? (b) Why does copper form at the negative electrode? (c) What would be seen happening at the positive electrode? (d) Carbon is unreactive and insoluble in water. Give another reason why it is suitable for use as electrodes (a) Electrolysis (b) Copper ions have a positive charge and are attracted to the negative electrode. Standard Grade Chemistry (c) Bubbles of chlorine gas given off. (d) Carbon is only non-metal element that conducts electricity. Unit 7 Revision. Q14. a) The elements in group 7 exist as diatomic molecules. i) What is meant by diatomic? ii) What type of bonding is present in a diatomic molecule? b) Information on group 7 elements is shown in the table. Name Atomic Boiling Number point / °C fluorine 9 –188 chlorine 17 – 35 bromine 35 59 iodine 53 184 astatine 85 ? i) What happens to the boiling point as the atomic number increases? ii) Predict the boiling point of astatine. (a) (i) A two atom molecule. (ii) Covalent bond between the atoms inside the molecule, and van der Waal forces between the molecules. (b) (i) Boiling point increases. Standard Grade Chemistry (ii) A value of between 275oCto 340oC Unit 7 Revision. Q15. The box below shows some atoms and ions. 24 14 Na 19 C 11 24 Mg 2+ F 6 9 12 19 12 F9 C 6 (a) Identify the two particles with the same number of neutrons (b) Identify the two atoms which are isotopes. (c) Identify the two particles with the same electron arrangement as neon. 19 (a) F 19 F- have 10 neutrons per atom and 9 9 14 12 (b) C and 6 C 6 24 Standard Grade Chemistry (c) are isotopes (same at, no, different mass no.) Mg2+ 12 19 and F- have the same number of electrons as neon 9 Unit 7 Revision. Q16. Write ionic formulae for (a) Potassium hydrogen carbonate. (a) K+HCO3 - (b) Ca 2+(NO3 -) 2 (b) Calcium nitrate. (c) Copper(II) permanganate. (c) Cu 2+(MnO4 -) 2 (d) Ba 2+(Cl-) 2 (d) Barium chloride. Q17. You are given the following information about 4 different substances. Substance Melting point (oC) Conductivity A 1700 Does not conduct when solid or molten B 686 Conducts when molten but not when solid C -73 D 649 Does not conduct when solid or molten Conducts when solid and when molten Which substance has the following bonding and structure? Standard Grade Chemistry (a) Covalent molecular. (a) C (b) Covalent network. (b) A (c) Ionic network (c) B (d) Metallic lattice (d) D Unit 7 Revision. Q. 18 Carbon and silicon are both elements in group 4 of the Periodic Table. Both elements form oxides. Carbon dioxide (CO2) is a gas at room temperature whereas silicon dioxide (SiO2) is a high melting point solid. Explain in terms of structure and bonding the differences between these two oxides. Carbon dioxide is covalent molecular with weak van der Waals forces between the molecules of carbon dioxide. As the van der Waals forces are weak this substance has a low melting and boiling point. Silicon dioxide has a covalent network structure composed entirely of strong covalent bonds. As the bonds are all strong this compound has a high melting point. Q. 19 Explain why solid lead bromide does not conduct electricity whereas molten lead bromide does. Standard Grade Chemistry In the solid state the ions in lead bromide are not free to move (they are held in an ionic network). When molten the ions are free to move and so can carry the current between the electrodes. Unit 7 Revision. Q. 18 A C + - + - + - + - - + - + B D Which of the structures, A to D, shown above represents (a) A covalent molecular substance (eg a water molecule) (b) A metallic lattice (eg sodium) (c) An ionic substance (eg sodium chloride) (d) A covalent network structure (eg carbon in diamond). (a) C (b) D (c) A (d) B