Chapter 11 Review (Slides)

Chapter 11: Chemical
Reactions
Test #2 Review
Balancing Equations
There are three steps to the process:
1.
Write the unbalanced equation (Skeleton equation).
2.
Balance the equation.
-Apply the Law of Conservation of Mass to get the same number of atoms of every element on each side of the equation. Tip: Start by balancing an element that appears in only one reactant and product.
-Once one element is balanced, proceed to balance another, and another, until all elements are balanced.
-Balance chemical formulas by placing coefficients in front of them. Do not add subscripts, because this will change the formulas.
3.
Indicate the states (phases) of matter of the reactants and products.
-Use (g) for gaseous substances.
-Use (s) for solids.
-Use (l) for liquids.
-Use (aq) for species in solution in water.
-Write the state of matter immediately following the formula of the substance it describes.
Example
Tin oxide (SnO) is heated with hydrogen gas (H
2 to form tin metal (Sn) and water vapor (H
)
2
O). Write the balanced equation that describes this reaction.
1.
Write the unbalanced equation (Skeleton equation).
SnO
2
+ H
2
→ Sn + H
2
O
2.
Balance the equation.
SnO
2
+ H
2
→ Sn + 2 H
2
O
Example (continued)
SnO
2
+ 2 H
2
→ Sn + 2 H
2
O
3. Indicate the physical states of the reactants and products.
SnO
2
(s) + 2 H
2
(g) → Sn(s) + 2 H
2
O(g)
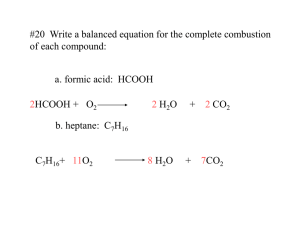
Balancing Equations (continued)
Problem: Balance the equation below
• C
7
H
17
(l) + O
2
(g)
CO
2
(g) + H
2
O (g)
Answer:
• 4 C
7
H
17
(l) + 45 O
2
(g) 28 CO
2
(g) + 34 H
2
O (g)
Classifying Chemical Reactions
1.
Synthesis (also called Combination):
A + B ----> AB or
(element or compound) + (element or compound) -----> compound
Example: CaO(s) + H
2
O(l) ----> Ca(OH)
2
(s)
2.
Decomposition:
AB ----> A + B or
Compound ------> (element or compound) + (element or compound)
Example: 2Ag
2
O(s) ----> 4Ag(s) + O
2
(g)
3.
Single Displacement (also called Single Replacement):
A + BC ----> B or
+ AC element + compound ----> element + compound
Example: 2Al(s) + 3CuCl
2
(aq) ---> 2AlCl
3
(aq) + 3Cu(s)
Classifying Chemical Reactions (continued)
4.
Double Displacement (also called Double Replacement):
AB + CD ----> CB + AD or
Compound + Compound ----> Compound + Compound
Example: AgNO
3
(aq) + NaCl(aq) ----> AgCl(s) + NaNO
3
(aq)
5.
Combustion: hydrocarbon + oxygen ----> carbon dioxide and water
Example: C
3
H
8
(g) + 5O
2
(g) ----> 4H
2
O(g) + 3CO
2
(g)
Classifying Chemical Reactions
Examples.
•
C
12
H
22
O
11
(s) ----> 12C(s) + 11H
2
O (g)
( Decomposition )
• H
2
SO
4
(aq) + 2NaOH(aq) ----> Na
2
SO
4
(aq) + 2H
2
O (l)
( Double Displacement )
Classifying Chemical Reactions (continued)
CH
4
(g) + 2O
2
(g) ----> 2H
2
O(g) + CO
2
(g)
( Combustion )
Zn(s) + H
2
SO
4
(aq) ----> ZnSO
4
(aq) + H
2
(g)
( Single Displacement )
2H
2
(g) + O
2
(g) ----> 2H
2
O(g)
( Combination/Synthesis or Combustion )
Classifying Chemical Reactions (continued)
C(s) + O
2
(g) ----> CO
2
(g)
( Synthesis/Combination or Combustion )
Pb(OH)
2
(s) ----> PbO(s) + H
2
O(g)
( Decomposition )
Classifying Chemical Reactions (continued)
2C
2
H
6
(g) + 7O
2
(g) ----> 6H
2
O(g) + 4CO
2
(g)
( Combustion )
ZnBr
2
(aq) + 2AgNO
3
(aq) ----> Zn(NO
3
)
2
(aq) + 2AgBr(s)
( Double Displacement )
Net ionic equations, formation of a precipitate, and identification of spectator ions
Ba(NO
3
)
2
(aq) + K
2
CO
3
(aq)
?
Ba(NO
3
)
2
(aq) + K
2
CO
3
(aq)
BaCO
3
(s) + 2KNO
3
(aq)
Total Ionic:
Ba +2 (aq) + NO
3
-1 (aq) + K +1 (aq) + CO
3
-2 (aq)
BaCO
3
(s) + K +1 (aq) + NO
3
(aq)
Spectator ions:
NO
3
-1 (aq), K +1 (aq)
Net Ionic: Ba +2
(aq)
+ CO
3
-2
(aq)
BaCO
3
(s)
Net ionic equations, formation of a precipitate, and identification of spectator ions (continued)
AgNO
3
(aq) + K
3
PO
4
(aq)
?
AgNO
3
(aq) + K
3
PO
4
(aq)
Ag
3
PO
4
(s) + KNO
3
(aq)
Total Ionic:
Ag + (aq)+ NO
3
(aq)+ K + (aq)+ PO
4
3(aq)
Ag
3
PO
4
(s) + NO
3
(aq) + K + (aq)
Spectator Ions: NO
3
(aq), K + (aq)
Net Ionic: 3Ag + (aq) + PO
4
3(aq)
Ag
3
PO
4
(s)