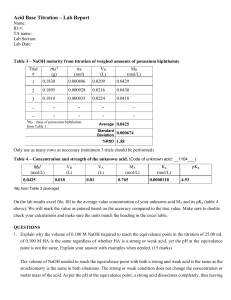
ACID-BASE TITRATION
advertisement

ACID-BASE TITRATION Experimental procedure used to establish the concentration of an acidic or basic solution when the values are unknown. The concentration of one solution is determined by quantitatively observing its reaction with a solution of known concentration (standard solution). The goal is to find the point at which the number of moles of the standard solution is stoichiometrically equal to the original number of moles of the unknown solution (the equivalence point). The end-point of the titration occurs when the indicator changes colour. The indicator is chosen so that it matches its equivalence point. Problem: What volume of 0.20 M soldium hydroxide is required to neutralize a 25.0 mL solution of 0.010 M sulphuric acid? Strategy: find the balanced chemical equation and find the moles of the acid. 2 NaOH + 1 H2SO4 Na2SO4 + 2 H2O Solution: nA = M x V nA = _____________________ nA = ___________ mol H2SO4 since NaOH and H2SO4 are in a 2:1 ratio nB = _______________ mol NaOH VB = nB/M VB = mol mol/L VB = ______________mL H2SO4 Shortcut: nA = nB MAVA = MBVB (0.020 M)(25 mL) = (0.20M) x x = _______ mL (caution – for the above calculation you must double the above molarity if it were a diprotic acid)