Half Lifes
advertisement

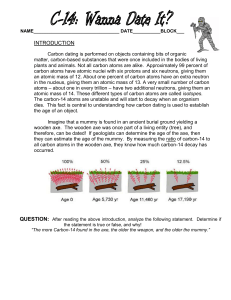
Radioactivity (Exponential Example) Atoms Atoms are made of protons, electrons and neutrons. Protons and neutrons reside in the middle, or nucleus, and electrons reside in the outer portion of an atom. Atomic Number The atomic number is the number of protons an element has. Hydrogen has one proton so its atomic number is 1 (see picture). Uranium has 92 protons so its atomic number is 92. Atomic Mass Number The atomic mass number indicates the total number of protons and neutrons in the nucleus For example, carbon has 6 protons so its atomic number is 6. Its atomic mass number, however, depends on the number of protons and neutrons it has. Carbon typically has an atomic mass number of 12. (6 protons + 6 neutrons = 12) Atoms can have isotopes or variations in their Atomic Mass Number. For example, carbon-14 is an isotope of carbon. Carbon-14 still has 6 protons but the 14 indicates its Atomic Mass Number is 14 (6 protons + 8 neutrons = 14) Radioactive Decay / Half life Atoms like carbon-14, don’t remain carbon-14 forever. They can change or decay into other isotopes or different elements naturally. We measure a thing called half life, which is the time it takes for half of a sample of atoms to decay or change into something else. Half life example Suppose we find a rock with a C-14 mass of 100 grams that is entirely carbon-14 Carbon-14 decays to nitrogen-14 with a half life of 5730 years So after 5730 years our rock will contain 50 grams of carbon-14 and 50 grams of C-14 N-14 nitrogen-14 After an additional 5730 years we’ll have 25 grams of carbon 14 and 75 grams of nitrogen-14 as half of our carbon-14 again decays C-14 N-14 Half life example If we found a rock that was 50% carbon-14 and 50% nitrogen-14, we can assume that it is 5730 years old. This assumes nothing has happened to the rock that would change this decay rate – (erosion, use by animals etc.) Since the atoms decay at a fixed percent rate this naturally lends itself to exponential problems.