Chapter 2 Chemistry comes alive
advertisement

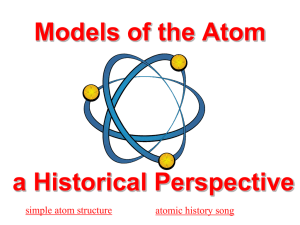
Section 2a Levels Chemical Cells Tissue Organ Organ system • Organism • • • • • Today we are working on… Matter • The “stuff” of the universe • Anything that has mass and takes up space • States of matter –Solid – has definite shape and volume –Liquid – has definite volume, changeable shape –Gas – has changeable shape and volume Energy • The capacity to do work (put matter into motion) • Types of energy –Kinetic – energy in action –Potential – energy of position; stored (inactive) energy Forms of Energy • Chemical – stored in the bonds of chemical substances • Electrical – results from the movement of charged particles • Mechanical – directly involved in moving matter • Radiant or electromagnetic – energy traveling in waves (i.e., visible light, ultraviolet light, and X rays) Energy Form Conversions • Energy is easily converted from one form to another • During conversion, some energy is “lost” as heat • Energy is never created or destroyed Composition of Matter • Elements are the fundamental units of matter • Elements – unique substances that cannot be broken down by ordinary chemical means Properties of Elements • Each element has unique physical and chemical properties –Physical properties – those detected with our senses –Chemical properties – pertain to the way atoms interact with one another Major Elements of the Human Body • There are about 120 known elements – 92 occur in nature – the rest are man-made • 96% of the body is made from four elements - Know these – • • • • Oxygen (O) Carbon (C) Hydrogen (H) Nitrogen (N) Lesser Elements of the Human Body • Lesser elements make up 3.9% of the body and include: –Calcium (Ca), phosphorus (P), potassium (K), sulfur (S), sodium (Na), chlorine (Cl), magnesium (Mg), iodine (I), and iron (Fe) Trace Elements of the Human Body • Trace elements make up less than 0.01% of the body –They are required in minute amounts, and are found as part of enzymes Composition of Matter • Each Element is composed of Atoms • Atoms = more or less identical building blocks for each element • Atomic symbol = one or two letter chemical shorthand for each element Carbon C Nitrogen N Oxygen O Calcium Ca Hydrogen H Sodium Na Atomic Structure • The nucleus consists of neutrons and protons –Neutrons – have no charge and a mass of one atomic mass unit (amu) –Protons – have a positive charge and a mass of 1 amu Atomic Structure • Electrons are found orbiting the nucleus –Electrons – have a negative charge and 1/2000 the mass of a proton (0 amu) Models of the Atom • Nucleus –Prontons (p+) –Neutrons (n0) • Outside of nucleus –Electrons (e-) Figure 2.1 Models of the Atom • Planetary Model – electrons move around the nucleus in fixed, circular orbits Figure 2.1 Models of the Atom • Orbital Model – regions around the nucleus in which electrons are most likely to be found Figure 2.1 Identification of Elements • Atomic number – –equal to the number of protons that the atoms contain • Mass number – –equal to the mass of the protons and neutrons – sum of the protons and neutrons Identification of Elements • Atomic weight – –average of the mass numbers of all isotopes –Close to mass number of most abundant isotope –Atomic weight reflects natural isotope variation Isotopes • Isotope – atoms with same number of protons but a different number of neutrons • Radioisotopes – atoms that undergo spontaneous decay called radioactivity Radioactivity • Rodioisotope – –Heavy isotope –Tends to be unstable –Decomposes to more stable isotope • Radioactivity –Process of spontaneous atomic Identification of Elements Figure 2.2 Identification of Elements Isotopes of Hydrogen Figure 2.3 Molecules and Compounds • Molecule – two or more atoms held together by chemical covalent bonds • Compound – two or more different kinds of atoms chemically bonded together in ionic bonds • Next time we will talk how these are chemically bonded Mixtures and Solutions • Mixtures – two or more components physically intermixed (not chemically bonded) • 3 basic types –Solutions –Colloids –Suspensions Solutions • Solutions – homogeneous mixtures of components –Solvent – substance present in greatest amount –Solute – substance(s) present in smaller amounts • May be gases, liquids, or solids Solutions • Water is the body’s chief solvent • Most solutions in the body are true solutions containing gases, liquids, or solids dissolved in water –True solutions are usually transparent Concentration of Solutions • True solutions are described in terms of their concentration (percent or molarity) • Percent, or parts per 100 parts • Molarity, or moles per liter (M) Concentration of Solutions • To make a one-molar solution of glucose – weigh out 1 mole of glucose and add enough water to make 1 liter of solution • A mole of an element or compound is equal to its atomic or molecular weight (sum of atomic weights) in grams • To find molecular weight of glucose C6 H12 O6 – Glucose has 6 carbon, 12 hydrogen, & 6 oxygen atoms • To compute molecular weight of glucose, look up the atomic weight of carbon, hydrogen and oxygen on a periodic table. • Molecular weight of glucose is 180.156 Atom # of atoms X Atomic weight = Atomic weight C 6 X 12.033 = 72066 H 12 X 1.008 = 12.096 O 6 X 15.999 = 95.994 180.456 Avogadro’s Number • One mole of any substance always contains exactly the same number of solute particles 6.02 X 1023 • So whether you weigh out 1 mole of glucose (180g) or water (18g) or methane (16g) or any other substance you will always have 6.02x1023 molecules of that substance Colloids and Suspensions • Colloids, or emulsions, are heterogeneous mixtures whose solutes do not settle out • Suspensions are heterogeneous mixtures with visible solutes that tend to settle out Mixtures Compared with Compounds • No chemical bonding takes place in mixtures • Most mixtures can be separated by physical means • Mixtures can be heterogeneous or homogeneous • Compounds cannot be separated by physical means • All compounds are homogeneous Quiz 1a over these lecture notes Study guide check pages 24-28