Atomic Structure Test Review - Chapter 4
advertisement

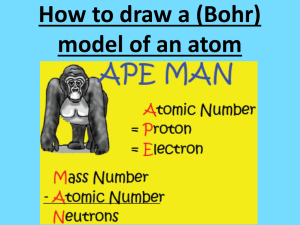
Fun in B208! Test review Chapter 4 Democritus thought that matter was made of tiny particles a. of earth, air, fire, and water. b. that could not be divided. c. that could be divided. d. that were all round and smooth. Which of the following is NOT part of John Dalton’s atomic theory? a. All elements are composed of atoms. b. All atoms of the same element have the same mass. c. Atoms contain subatomic particles. d. A compound contains atoms of more than one element. John Dalton’s model of the atom states a. a tiny, solid sphere with an unpredictable mass for a given element b. a hollow sphere with a dense nucleus c. a tiny, solid sphere with predictable mass for a given element d. a sphere that is hollow throughout J.J. Thomson’s experiments proved that an atom a. is the smallest particle of matter. b. has a negative charge. c. contains negatively charged particles. d. has a positive charge. In Rutherfords experiment, what caused some of the alpha particles to bounce straight back from the gold foil? a. electrons in the gold atoms b. other alpha particles c. negative charges in the gold atoms d. nuclei in the gold atoms In Niels Bohr’s model of the atom, electrons move a. like balls rolling down a hill. b. like popcorn in a popcorn popper. c. like planets orbiting the sun. d. like beach balls on water waves. Bohr’s model has _______ move in fixed orbits around the nucleus. A. neutrons B. electrons C. protons D. atomic mass number Which subatomic particle has a negative charge? a. electron b. neutron c. alpha particle d. proton Which statement about subatomic particles is true? a. Protons, neutrons, and electrons all have about the same mass. b. Unlike protons or neutrons, electrons have no mass. c. Neutrons have no charge and no mass. d. An electron has far less mass than either a proton or neutron. Which of the following is unique for any given element? a. the number of neutrons b. the number of protons c. the charge on the electrons d. the mass of a neutron The number of protons in one atom of an element is that element’s a. mass number. b. atomic number. c. balanced charge. d. isotope. To find the number of neutrons in an atom, you would subtract a. mass number from atomic number. b. atomic number from electron number. c. atomic number from mass number. d. isotope number from atomic number. The nuclei of isotopes contain different numbers of _________. A. neutrons B. electrons C. protons D. atomic mass number The atomic number refers to the number of _____ in an atom. A. neutrons B. electrons C. protons D. atomic mass number The atomic mass refers to the number of _____&_____ . A. neutrons B. electrons C. protons D. atomic mass number Complete the table. Protons Sodium - 24 Lithium - 7 Aluminum - 27 11 3 13 Neutrons Electrons 13 4 14 11 3 13