Exponential Functions Review - AP Calculus AB
advertisement
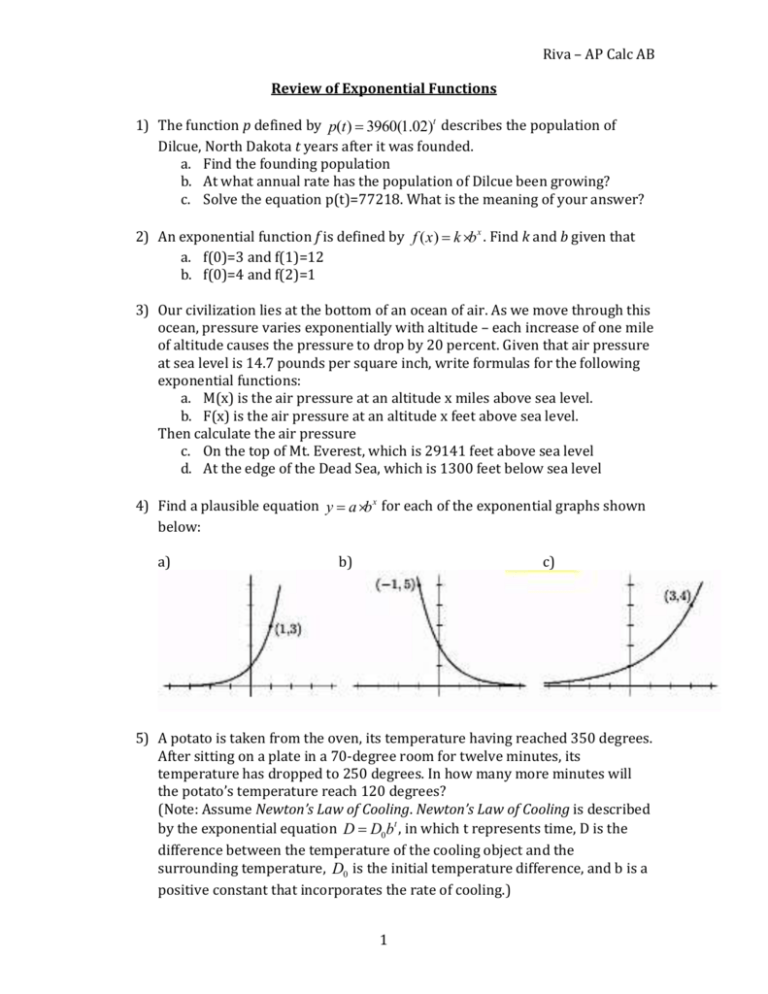
Riva – AP Calc AB Review of Exponential Functions 1) The function p defined by p(t) = 3960(1.02)t describes the population of Dilcue, North Dakota t years after it was founded. a. Find the founding population b. At what annual rate has the population of Dilcue been growing? c. Solve the equation p(t)=77218. What is the meaning of your answer? 2) An exponential function f is defined by f (x) = k × b x . Find k and b given that a. f(0)=3 and f(1)=12 b. f(0)=4 and f(2)=1 3) Our civilization lies at the bottom of an ocean of air. As we move through this ocean, pressure varies exponentially with altitude – each increase of one mile of altitude causes the pressure to drop by 20 percent. Given that air pressure at sea level is 14.7 pounds per square inch, write formulas for the following exponential functions: a. M(x) is the air pressure at an altitude x miles above sea level. b. F(x) is the air pressure at an altitude x feet above sea level. Then calculate the air pressure c. On the top of Mt. Everest, which is 29141 feet above sea level d. At the edge of the Dead Sea, which is 1300 feet below sea level 4) Find a plausible equation y = a × b x for each of the exponential graphs shown below: a) b) c) 5) A potato is taken from the oven, its temperature having reached 350 degrees. After sitting on a plate in a 70-degree room for twelve minutes, its temperature has dropped to 250 degrees. In how many more minutes will the potato’s temperature reach 120 degrees? (Note: Assume Newton’s Law of Cooling. Newton’s Law of Cooling is described by the exponential equation D = D0 bt , in which t represents time, D is the difference between the temperature of the cooling object and the surrounding temperature, D0 is the initial temperature difference, and b is a positive constant that incorporates the rate of cooling.) 1 Riva – AP Calc AB 6) An atom of carbon-14 is unstable, meaning that it can spontaneously transfer itself (by radioactive decay) into nitrogen at any instant. The probability that this will actually happen to a specific atom of carbon-14 in the course of a year is only about 0.0121 percent, however. In other words, there is a 99.9879 percent chance that any specific atom of carbon-14 will survive another year. Suppose that one million carbon-14 atoms are placed in a container. a. How many of these atoms are expected to be carbon-14 atoms one year later? b. The half-life question: How much time will it take for half of the atoms to decay? 7) (continuation) Carbon-14, which is produced from nitrogen when solar radiation bombards the upper atmosphere, makes up about 1 trillionth of all the carbon found in things that rely on air to live. When an organism dies, it no longer takes in air, and its supply of carbon-14 diminishes exponentially as described above. Apply this principle to estimate the age of a lump of charcoal (found in a cavern at an ancient campsite) that has only 32 percent as much carbon-14 as it had when the charcoal was still firewood. 2 Riva – AP Calc AB Answers 1) a. 3,960; b. 2% increase; c. 150 years x 1ö æ x 2) a. f (x) = 3× 4 ; b. f (x) = 4 × ç ÷ è 2ø 3) a. M(x) = 14.7×(.8)x ; b. M(x) = 14.7×(.999958)x ; c.4.29 lb per sq in; d. 15.53 lb per sq in x æ 2ö 4) a. y = 1×3x ; b. f (x) = 2 × ç ÷ ; f (x) = 1× è 5ø 5) 46.8 minutes 6) a) 999879; b) 5728 years 7) 9,416 years ( 4) 3 x 3