dr. sn naik
advertisement

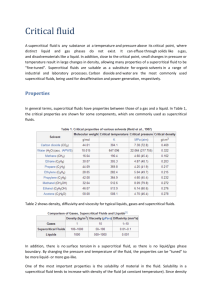
Supercritical fluid Extraction and it’s Applications Dr S. N. Naik Center For Rural Development and Technology IIT Delhi Outline • • • • • • Supercritical fluid as a solvent Application of supercritical fluids Supercritical carbon dioxide extraction of botanicals Particle design using supercritical fluid Biomass conversion using Supercritical fluid SCF based Bio refinery Supercritical fluid as a solvent • Solvent are used in large amount in chemicals, pharmaceutical, food and natural products industry • In search of environmental friendly solvent, attentation has been paid to supercritical fluid for wide application in extraction,chromatograhy, particle design, reaction, drying etc. Physical properties of gas ,liquids and supercritical fluids Typical Supercritical Solvents Compound Tcº C Pc atm d* CO2 31.3 72.9 0.96 C2H4 9.9 50.5 --- N2O 36.5 72.5 0.94 NH3 132.5 112.5 0.40 n-C5 196.6 33.3 0.51 n-C4 152.0 37.5 0.50 CCl2F2 111.8 40.7 1.12 CHF3 25.9 46.9 ---- H2O 374.1 218.3 ---- Advantages of SCF Application of SCF Extraction of value added Chemicals from Biomass Bioactive compounds from Botanicals Particle design using supercritical fluid • Pharmaceutical, Nutraceutical, Cosmetic, Specialty chemistry industry • • RESS: Rapid Expansion of Supercritical Solutions Consist in solvating the product in the fluid and rapidly depressurizing this solution through an adequate nozzle(<100micro m to 20 micro m diameter) • Attractive due to the absence of organic solvent use • • Its application is restricted to products that present a reasonable solubility in supercritical carbon dioxide(low polarity compounds) • • GAS or SAS: Gas(or Supercritical fluid) Anti-Solvent Consist in decreasing the solvent power of a polar liquid solvent in which the substrate is dissolved, by saturating it with carbon dioxide in supercritical conditions, causing the substrate precipitation or recrystallization • • Rapid expansion of supercritical solutions (RESS) • Depressurizing this solution through a heated nozzle into a low pressure chamber in order to cause an extremely rapid nucleation of the substrate(s) in form of very small particles • In the precipitation unit, the supercritical solution is expanded through a nozzle that must be reheated to avoid plugging by substrate(s) precipitation • The morphology of the resulting solid material both depends on the material structure crystalline or amorphous, composite or pure. • The RESS parameters; • temperature, pressure drop, distance of impact of the jet against the surface, • dimensions of the atomization vessel, nozzle geometry RESS Process • RESS is a very attractive process as it is simple and relatively easy to implement at least at small scale when a single nozzle can be used. • Extrapolation to a significant production size requires either a multinozzle system or use of a porous sintered disk through which pulverization occurs, • In both the case, particle size distribution is not easy to control and may be much wider than in the case of a single nozzle. • Particle harvesting is complex • The most important limitation of RESS development lies in the too low solubility of compounds in supercritical fluids • In most cases, use of a co-solvent to increase solubility in the fluid is not feasible Supercritical anti-solvent and related process (GAS/SAS) • In this process, the supercritical fluid is used as an anti-solvent that causes precipitation of the substrate(s) dissolved initially in a liquid solvent. • A batch of solution is expanded several-fold by mixing with a dense gas in a vessel. • Due to the dissolution of the compressed gas the expanded solvent has a lower solvent strength than the pure solvent. • The mixture becomes supersaturated and solute precipitates in microparticles. • This process has been called gas anti-solvent(GAS) or supercritical anti-solvent(SAS) recrystallization GAS/SAS Antisolvent Process • Spraying the solution through an atomization nozzle as fine droplets into compressed carbon dioxide. • The dissolution of the supercritical fluid into the liquid droplets is accompanied by a large volume expansion and, consequently a reduction in the liquid solvent power causing a sharp rise in the supersaturation within the liquid mixture and the consequent formation of small and uniform particles. This spray process has been called Aerosol solvent extraction system (ASES) process ASES Process Biomass conversion using Supercritical fluid Cellulose degradation pathways by SCW Dielectric constant and Density of Water at 1000 C Ion product of Water Hydrolysis of cellulose in SCW Potential reaction products from the decomposition of Lignin Bio refinery • Bio refinery involves sustainable processing of biomass into a spectrum of value added products • Bio-based chemicals, materials, food, feed etc. • Bio energy (biofuels, power and heat) • Bio refinery value chain: Biomass production, conversion, recycling, conformity of end products to user requirement Bio-refinery Secondary metabolites (terpenoids, alkaloids,lipids) Biomass Hemicellulose Cellulose Lignin Ash Bio-fuels and Chemicals Supercritical Fluid based Biorefinery Advantages of Bio-refinery • Conservation of fossil resources • Renewable resources are CO2 neutral • Products are bio-degradable • Raw materials are non-toxic Producing chemicals from bio-mass requires newer clean technology Extraction of Minor Constituents Waxes COSMETICS, COATINGS, ETC.. Polycosanols/ sterols Long chain alkanes OH n HO CHOLESTEROL REDUCING AGENTS n INSECT SEMIOCHEMICALS Major Constituents conversion HEMICELLULOSE (25-35%) OH H O HO HO H OH H O H H H H HO H OH O HO H H OH OH H OH HO OH H HO H H H OH OH Ethanol, lactic acid,furfural derivatives, glucose, xylose D-glucose D-galactose D-Mannose O H Energy H H HO HO H H H O HO H OH D-xy lose OH H H HO OH H H O OH H H OH D-arabinose O HO HO H H H OH D-glucuronic acid LIGNIN CELLULOSE Vanillin and analogues PAPER, strawboard (15-20%) (45-55%) products, plastics MINOR CONSTITUENTS (5-10%) OH H Production of biooil via fast pyrolysis process Feedstock Biooil Char Quench liquid Recycled gases Feedstock Cyclone/ Char Collector Pyrolysis reactor Quench system Bio-oil storage Wood Bio-oil 100 cm3 of wood contains the same energy as 20 cm3 of Bio-oil SC-CO2 Extraction of Bio-oil (set-up and samples) Up gradation of Bio-oil using SCF Biooil as such can not be used as transportation fuel as it contains high percentage of water (~ 45 wt %). Also, water decreases its calorific value, It forms azeotrope with organic compounds, It increases percentage of oxygen and results in polymerization at room temperature in few weeks. Methods for removal of water: Solvent extraction Supercritical CO2 extraction (Green process) Proximate analysis and calorific value of mixed biomass (wt. %) wt % Moisture Content Ash Content Volatile Content Fixed Carbon 8.3 1.8 83.0 6.9 Measurement error : ± 0.2 Calorific value = 18.6 MJ/kg Chemical Analysis of mixed Biomass C/H/N/S/O analysis results wt % C H N S O 46.70 6.20 0.07 0.05 46.00 Ca Al P ICP-MS of major compounds in ash Fe ppm Mg 59,491 13,300 74,430 39,111 5,787 Composition of Biomass* • Hemi-cellulose 34.1 ± 1.2 wt % • Cellulose 44.4 ± 1.4 wt % • Lignin 21.5 ± 1.0 wt % * Calculated using acid (dilute H2SO4) –process and HPLC analysis Comparative XRD analysis of mixedbiomass, cellulose and lignin Intensity a: biomass b: lignin c: cellulose 2Ф Biomass has crystalline character due to the presence of cellulose m (μg) Comparative TG Analysis of biomass, cellulose and lignin T (oC) The range of main weight loss for biomass is 200-550 oC. DTG (μg/min) Comparative DTG Analysis of biomass, cellulose and lignin T (oC) 100oC< loss of easily volatiles, 100-130oC (water), 250-320oC (hemicellulose), 300-400oC (cellulose) and 250-750oC (lignin) HPLC Analysis (NREL Method) of structural Carbohydrate in Biomass wt % Glucose Xylose Galactose Arabinose Mannose 56.4 ± 0.2 6.3 ± 0.5 1.9 ± 0.1 31.8 ± 0.7 3.6 ± 0.6 Supercritical CO2 Extraction process of bio-oil produced from mixed biomass • 50 g of bio-oil was mixed with 2 mm glass beads and placed in the extractor to half of its volume. • Extraction was carried out at 40oC with CO2 flow rate of 40 mL/min • The first fraction was collected at 10 MPa (100 atm) and was continued for 2 h • Then pressure was raised to 25 MPa and the extraction was continued for 2 h to collect the second fraction. • The remaining oil was again treated in the same way at 30 MPa to collect the third fraction. Conclusions • SC-CO2 extraction process can be used for separation of bioactive compounds from botanicals. • Supercritical fluid based biorefinery can be used to produce value added chemicals and 2nd generation biofuel from lignocellulosic biomass • The upgraded bio-oil can be used for production of clean fuel. • SCW can be used for conversion of lignocellulosic biomass to water soluble fermentable sugars for production of bio-ethanol and chemicals. Email: snn@iitd.ernet.in