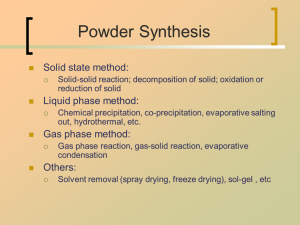
Basic principle of l..
advertisement

Che5700 陶瓷粉末處理
Introduction to Liquid Phase
Synthesis
Very common; simple; cheap;
Easy to get multi-component product, high uniformity,
dispersion in atomic scale;
Often more steps; complex inter-relationship; often
need calcination to get final useful product
Classification: (the way to remove solvent)
Solvent evaporation: spray drying, spray
decomposition, evaporative decomposition of solutions
EDS; emulsion drying, freeze drying
Precipitation-filtration: ordinary process; homogeneous
precipitation
Solvent extraction: salting out; sol-gel (?)
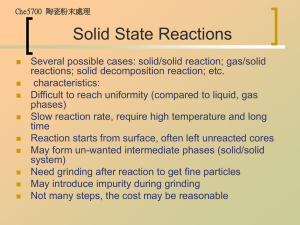
Che5700 陶瓷粉末處理
Process Introduction
Precursor in solvent (aqueous or organic) one or
several precursors chemical reaction (additive,
temp., etc.) separation from solvent postprocessing (washing, drying, etc.) product powder
Precipitation method: co-precipitation, homogeneous
precipitation, emulsion precipitation, hydrothermal
precipitation, hydrolytic precipitation (referring to solgel, alkoxide was hydrolyzed)
Important parameters: pH, temperature, time,
precipitation agent, quantity, rate of addition, method
of addition, type of cation, type of solvent and quantity,
reactor size and shape, other additives, stirring,
atmosphere and pressure (e.g. in autoclave) VERY
COMPLEX; often rely on experimental design
UO2 nuclear fuel
rod material;
Reaction: UO2F2
+ (NH4)2CO3
(NH4)4UO2(CO3)3
+ 2 NH4F
Complex steps
experimental
design to find
optimal
condition quickly,
e.g. Taguchi
method,
Plackett –
Burman etc.
•Top figure: a,b,c –
particle size distribution
after precipitation,
washing/drying and
calcination, agglomeration
during washing and
drying is obvious
•Bottom figure: relation
between average size
(after calcination) and
sintered density; only
qualitative in nature.
* Taken from JS Reed; precipitation occurs when two
chemical reacting with each other, formation of particles –
described by the theory of nucleation and growth
Che5700 陶瓷粉末處理
Expression of supersaturation
supersaturation: C = C – C or m, x
Supersaturation ratio: = C/C
Relative supersaturation: C = C/C; x + 1 = x/x
Dimensionless growth affinity: = /RT
For Activity & activity coefficient of ions: thermodynamic
equations such as Debye-Huckel equation
i (i i ) & i i RT ln ai
eq
o
ai i xi & xi i x
{1 ln( / ) / ln(x / xeq )}RT ln(1 x )
eq
Che5700 陶瓷粉末處理
Electrolytic Solutions
• Behavior of ions: non-ideal solution; due to strong
interaction between ions
• electrical neutrality: z+ NA + z- NB = 0 (Aν+Bν- = ν+
A z+ +ν- Bz-; ν+ z+ +ν- z- = 0;)
• mean ionic activity coefficient: γ±ν = (γA□) ν+ (γB□) νwhere ν=ν+ +ν• mean ionic molality: M±ν=MA ν+ MB ν• Debye-Huckel limiting law: ln γ± = - α∣z+ z-∣√I;
where I = ionic strength = ½ Σ zi2 Mi (over all ions);
α: parameter of system = f(T, solvent) (find it out in
handbooks for common solvents)
=RT ln; = (i/Ksp) 1/; = i
Ksp: ionic product at equilibrium; i = current ionic
product; ratio of these two values ~ supersaturation
Solubility
Thermodynamic data: mainly affected by
temperature, and solution environment (e.g. other
ions, pH,…)
a
G ( 2 1 ) RT ln( )
ao
C
a
S
Co ao
Solubility (2)
Temp. & pH effect: DCP =
dicalcium phosphate; HAP =
hydroxyapatite;
System of: Ca(OH)2-H3PO4 –
KOH – HNO3 – CO2 – H2O;
Ca/P = 1
2
A 2 B AB2( s )
2
2
o
K sp [ A ]o [ B ]
2
2
S [ A ][B ] / K sp
• ΔT: also used as a
measure of
supersaturation (as
shown in figure);
•Solubility often
increase with
temperature; (there are
also contrary cases, e.g.
CaCO3 solubility in
water decrease with
temperature; the reason
we get “scales”)
Che5700 陶瓷粉末處理
Nucleation
Several cases: homogeneous nucleation, heterogeneous
nucleation, secondary nucleation
For homogeneous nucleation: for its rate, we have
thermodynamic model or kinetic model
Thermodynamic model: changes between surface energy
and bulk energy, energy of formation of new crystals
= Ac – ( - ) Mc [Ac: crystal surface area; Mc:
crystal mass) when nucleus size reach some critical
value d /d(d) = 0 to get critical nucleus size d* =
4 Vm /(RT ln)
Finally to derive the rate equation:
Bo = C exp(- */kT) & * = 32 b 3 Vm2/(RT ln)2
S=
Che5700 陶瓷粉末處理
Kinetic Expression of Nucleation
Kinetic viewpoint: A1 + A1 = A2 + A1 = A3 …. A i+1 +..
A1 = monomer Then the following kinetic equations
Ci = condensation rate; Ei = evaporation rate
Under steady state d fi/dt = 0, and B.C. f1 = n1 =
constant; fG = 0 or constant (G: some critical size, e.g.
critical nucleus size)
dfi / dt Ci 1 fi 1 Ei fi Ci fi Ei 1 fi 1 I (i 1, t ) I (i.t )
I ao P / 2mkT(ao / 9kT )
1/ 2
I Z (i*)C (i*)n(i*)
exp(4ao 3 / 27k 3T 3 (ln P / Pe )2 )
3
Zeldovich factor
Che5700 陶瓷粉末處理
Solute Clustering & Nucleation
Taken from JCG, 89, 202-208, 1988.
Main viewpoint: solute molecules aggregate to form
clusters (precursor to nuclei), surface energy of cluster
may differ from large particles (different structure).
At 0oC, for water, 76% exist as clusters
One method to study cluster size and conc. : let
supersaturated solution stand for very long time
develop spatial distribution of clusters of different size,
measurement by density or opacity difference.
Indirectly, width for metastable zone, provide
information on cluster (narrow: cluster already exist,
easy to nucleation)
Typical cluster size: 4-10nm, ~ 103 molecules
Che5700 陶瓷粉末處理
Heterogeneous Nucleation
Reasons to heterogeneous nucleation:
larger complex size; impurity; wall of container;
liquid/air interface
Due to lowering of surface energy, (lowering
barrier to nucleation)
In a sense, co-precipitation: similar effect
Epitaxial growth: similar structure between nuclei
and impurity surface, therefore growth of nuclei
on this impurity surface
Used to make core-shell particles, core as seed
to shell particles
Complex ions
can increase size
of cluster, closer
to critical
nucleus size,
helpful to
nucleation;
Impurity also
influence
structure (phase)
of product
Che5700 陶瓷粉末處理
More on Nucleation
Taken from
TA Ring,
1996; data
for BaSO4
Che5700 陶瓷粉末處理
Secondary Nucleation
Under-saturated condition, existing nuclei
induce new nucleation – secondary nucleation
Reasons include:
Initial breeding;
Needle breeding
Contact breeding; Fluid shear etc.
parameters: degree of supersaturation, stirring,
collision between suspending particles
(frequency, energy, material of container etc)
Empirical relation: secondary nucleation rate Bo
~ (S-1)b MTj (rpm)h; where MT = quantity of
suspending particles
A Model on Secondary Nucleation
Taken from Botsaris, et. al. Chem. Eng. Sci., 52(20), 34293440, 1996;
Their concept: in supersaturated solution, existing embryos
(may be viewed as a result of coagulation between clusters),
they aggregate (due to van der Waals forces attractive forces),
if also seed, embryos move to seed, in the neighbor of seed:
high embryo concentration, they will aggregate to form new
nuclei, swept by fluid to become secondary nuclei, some may
aggregate with seed to make it bigger
Theory of rapid coagulation: - dn/dt = 8D r n2 = (4kT/3) n2
(by Smoluchowski) (particle movement by Brownian motion; n:
particle conc. r particle radius; D diffusion coefficient)
Botsaris: estimate secondary nucleation rate near a seed;
curve 7: assume cluster g = 622; At = seed surface area = 1.67
cm2/cm3; system: KCl-H2O; curve 6 first half: contact
nucleation; second half: similar to Botsaris’ theory
LH left-hand
左旋光結構
To demonstrate relation between seed and nuclei: use chiral
compound; low supersaturation: some effect, middle:
significant; high supersaturation: homogeneous nucleation
This impurity
show
inhibiting
effect
Che5700 陶瓷粉末處理
Induction Times
•
From start of generation of supersaturation until
observation of crystals, - induction time
• Techniques to observe crystals: turbidity, visual
observation, conductivity, or properties related to
concentration
• It include three parts:
ti = tr + tn + tg
ti : induction time;
tr: time required for attainment of stationary embryo
distribution (relaxation time)
tn: time for the formation of nucleus
tg: time for nucleus to grow into detectable crystals
* One possible barrier to nucleation: dehydration reaction of
ions
Che5700 陶瓷粉末處理
More on Induction Times
If tn: major part, nucleation dominate, tn ~ 1/Bo then ln(tn) or ln(ti)
vs ln() -2 should be linear
If tg: major, ti often becomes very long, its growth may be limited by
surface nucleation ln(ti) vs ln() -1 will be linear
sometimes, embryo
structure differs from
crystal, phase
change may become
barrier
Che5700 陶瓷粉末處理
Crystal Growth
Crystal growth: mass transfer, heat transfer can not be
neglected; species entering structure, may also the rate
determining step
Relation between size and solubility: OstwaldFreundlich law, similar to Kelvin equation; small size (L
small) high solubility
surface nucleation mechanism birth and spread
screw dislocation mechanism
Impurity effect: often inhibit growth by adsorption on
specific site (surface), often change morphology
ln(X / X eq ) 2M / 3LRT
•Growth steps:
•Diffusion to surface; Adsorption; Desolvation;
(dehydration); Surface diffusion; Integration at kink site
•terminology: ledge, step and kink
F = surface energy
xL/ xeq = a
measure
of supersaturation
It shows small size, large solubility; for low surface energy,
size effect less significant (see Kelvin equation)
Che5700 陶瓷粉末處理
Growth Rates
Different mechanism, different equations to show
relation between growth rate and supersaturation: e.g.
Birth & Spread mechanism (2D nucleation model):
growth rate ~ (step height) x (step velocity) 2/3 x
(#critical nuclei formed/area-time) 1/3 G = A i 5/6
exp(-B/i)
General empirical equation: G = k n
Note: can be supersaturation with respect to bulk, or
to surface
Can be
classified as:
linear, parabolic,
and
exponential law
(growth rate
and
supersaturation)
Che5700 陶瓷粉末處理
More on Growth Rates
Growth rate may depend upon size, I.e. G = f(L)
Growth rate dispersion: due to different residence time,
or due to surface structure & perfection
Too fast growth rate, easy to trap mother liquid
(inclusion)
Heat production: interface temperature may affect
solubility near interface i.e. super-saturation, or
growth rate
In general: linear growth rate = mass transfer or
adsorption effect
parabolic rate = spiral steps
exponential rate = polynuclear surface control
(H / M )GC hi (Ti T )
Growth rate
proportional to
density of
defects (screw
dislocation)
Accumulation of supersaturation nucleation
supersaturation decrease nucleation stops growth
continue end
Summary on Particle
Formation
Reaction formation of some “species”
supersaturation (induction times)
Nucleation (home-, hetero- ..) (critical nucleus
size, nucleation rate)
growth (growth rate, crystal habit, …)
agglomeration
final particle size distribution and morphology
Veiled: 蓋面紗的, 遮蔽的
Crystal Habit
Equilibrium shape versus growth shape
Former: surface energy of each surface
Latter: relative growth of each surface,
depending on growth environment
Equilibrium shape: ( Wulff theorem: following
equation) large surface energy, small surface
area, I.e. easy to disappear
S / L (1) A(1) S / L (2) A(2) ... const.
equilibrium
shape;
Elimination of high
energy surface via
growth
Different morphology: obtained under different
supersaturation (AgBr);
From octahedron (only 111 surface), gradually change
to tetradecahedron (showing 100 surface), finally to
cubic (with only 100 surfaces)
Taken from TA
Ring, 1996; by
adsorbing
impurity species
to control
morphology
Che5700 陶瓷粉末處理
Ostwald Ripening
An aging process, often cause coarsening of
large particles at the expense of small ones
Driving force: difference between solubility
between sizes (thermodynamically-driven);
Gibbs-Thompson equation; also influenced by
mass transfer and growth kinetics
Cs (r ) C C [exp(a / r ) 1]
a 2VM /RT
(from Wikipedia)
* Ostwald ripening (often water-inoil system) vs flocculation (oil-inwater system)
* Diffusion is often rate controlling
process
Oil droplet in pastis
mixed with water grow
by Ostwald ripening
Time progress of supersaturation with time; critical nucleus
size for each supersaturation, the particle will grow up in size,
but particles formed later may be consumed due to Ostwald
ripening effect
Taken from 游佩青博士論
文稿 (成大資源工程系;
p.16; 2008)
* Maximum growth rate
size ~ 2 x average size
(where growth rate = zero)
a3 – ao3 = [6 D co
γM/(ρ2RT)] (t – to) where
a = average size, D =
diffusion coefficient; co =
solubility at interface;
γ=interfacial energy;
Digestive Ripening
Source: Langmuir, 2002, 18, 7515-7520
* Transform polydispersed particle to monodispersed Au
colloids after ageing in the presence of dispersive agents
* These monodispersed
colloids may be stable only at
high temperature, will form
ordered precipitate when
temperature cools down;
Taken from: New Journal of Chemistry, 35, 755-763, 2011
•Possible mechanism: dispersion molecule (surfactant) may
penetrate large colloids to adsorb on the surface and to
create small colloids;
• When the chain length of dispersion molecule decrease,
the attractive force between two particle will increase and
tend to form precipitate (self-assemble into 3D superlattice – look like reversible crystallization);