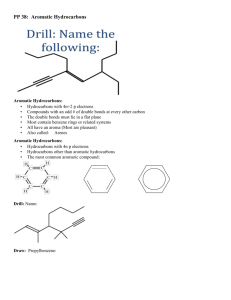
Aromatic hydrocarbons
advertisement

Aromatic hydrocarbons Unit 2: world of carbon HIGHER CHEMISTRY Aliphatic hydrocarbons • Where carbon atoms are linked together to form a chain. • Alkanes, alkenes & alkynes Alicylcic hydrocarbons • Carbon atoms are linked together to form rings. • Cycloalkanes, cycloakenes Aromatic hydrocarbons • Contain a ring of 6 carbon atoms. • The simplest compound is benzene C6H6 Structure of benzene Benzene • Carbon has 4 outer electrons. • 3 of these electrons are involved in covalent bonding with the 2 adjacent carbon atoms and the 1 hydrogen. • The last electron enters a pool of delocalised electrons. Uses • Benzene is an important feedstock to industry. • Many consumer products are manufactured using benzene as a feedstock. Halogenalkanes Uses • Trichloromethane, CHCl3, more commonly known as chloroform was used as an anaesthetic. • However it is known to damage the liver. CFC’s • Chlorofluorocarbons were developed for use where a non-flammable volatile liquid was required. • During the 80’s there was growing concern over the depletion of the ozone layer. CFC’s were resposible. • When UV rays met the CFC’s in the upper atmosphere the molecules would break up leaving a Cl atom. • This Cl atom then converts O3 (ozone) into O2. World of Carbon Fuels Unit 2 Fossil fuels - What are they? - How were they formed? - Fractional distillation Pollution - Greenhouse effect - Renewable / non renewable energy Hydrocarbons - Alkanes - Alkenes - Cycloalkanes Hydrocarbons - Isomerism - Saturation / Unsaturation - Cracking Petrol •This petrol fraction is not Ready to be used as a fuel. • Straight chain hydrocarbons do not perform well as fuels In petrol burning engines. •You can improve performance by adding aromatic & branched chain hydrocarbons Aromatic & branched Hydrocarbons • Aromatic hydrocarbons contain rings of 6 carbon atoms. • Branched-chain hydrocarbons are not straight chains. E.g. Reforming • Straight chain hydrocarbons undergo rearrangement to form branched chain hydrocarbons, without necessarily changing the number of C. CH3 CH3 CH3 CH2CH2CH2CH2CH2CH2CH3 CH3CCH2CHCH3 CH3 Refoming • Passing naphtha over a catalyst ( platinum or molybdenum (VI) oxide at about 500°C. • Straight chain alkanes may also be converted to aromatic hydrocarbons; Aromatic hydrocarbons • The Naptha fraction is an important feedstock for the production of aromatic hydrocarbons. • Cyclohexane (present in naphtha) can undergo dehydrogenation to produce benzene. Benzene • Benzene is the simplest aromatic hydrocarbon. • This process is known as reforming C6H12 C6H6 + 3H2 Benzene • Ring structure. Each corner of the hexagon represents a carbon atom with a hydrogen atom attached. • Toluene (methylbenzene) has one of these hydrogen atoms replaced by a CH methyl group. 3 C6H5CH3 Methyl benzene Making petrol • Products made from reforming naptha are combined with products of cracking longer chain hydrocarbons to produce more efficient fuel. • Butane is dissolved in petrol in the winter, this is to make it more volatile. Petrol engines • In a petrol engine, air and petrol vapour are drawn into the cylinder, compressed and ignited by a high voltage electric spark. Controlled explosion of petrol air mix forces piston down Knocking • Air-fuel mixture auto ignites or pre-ignites when the engine is hot. • It can be heard as ‘knocking’ or ‘pinking’ and is potentially damaging to the engine and inefficient. • Leaded petrol contains anti-knocking agent, lead tetra-ethyl Pb(C2H5)4 – toxic gases. • Unleaded petrol requires a much higher proportion of branched and aromatic hydrocarbons to increase the efficiency of burning. Polymers From previous work you should know the terms: 1. 2. 3. 4. 5. 6. 7. Alkenes Addition reaction Polymerisation Monomer Polymer Thermoplastic Thermosetting plastic Early plastics & fibres • Ethene & propene are very important starting materials in the petrochemical industry. Especially production of polymers. – Where do these starting materials come come from? – How can we produce more of them? Approximately 80% of ethene comes from cracking ethane Addition polymerisation Condensation polymers • Made from a monomer which contains 2 functional groups per molecule. • The monomers join in chains by eliminating a molecule of water. • Examples we will learn about include polyesters, polyamides and aramids. Polyester… • Is synthesised by condensation of 2 monomer units. • The first has 2 hydroxyl groups, know as a dialchohol. • The second has 2 carboxylic acid groups called a dicarboxylic acid. Hexanedioic acid Polyester 2H2O New ester links Uses of polyesters • If the monomers link only linearly, the polyester produced can be used for textile fibres, ropes, cords and sailcloth. • However if the monomers have additional hydroxyl (-OH) and carboxyl (-COOH) crosslinking occurs. • This creates a 3D thermoplastic used in the manufacture of bottles for soft drinks & fleece jackets. Hydrogen bonds in kevlar Proteins Proteins • Proteins are natural products formed by many plants and all animals. • All proteins contain carbon, hydrogen oxygen & nitrogen. • Nitrogen comes from either the air, fertilisers or from the action of bacteria in nodules on some plant routes. • Proteins are condensation polymers made by linking together many amino acids. Proteins • Different proteins are used for different processes in the body. • The body cannot produce all the amino acids required for body proteins. • Therefore essential amino acids must come from dietary proteins. Digestion • During digestion protein molecules are broken down or hydrolised to produce a mixture of amino acids. This process is catalysed by enzymes. • The amino acid can then pass through the gut wall into the bloodstream. Amino acids.. • As the name suggests; contain both an acid group • And an amino group (NH2) H O N H R C OH Condensation polymerisation Peptide link Hydrolysis of proteins • The structural formula of the amino acid released from the protein can be identified by looking at the corresponding section of the protein chain. • In nature, hydrolysis of proteins is catalysed by enzymes. • In the lab, hydrolysis of proteins is catalysed by dilute acid or alkali. Helix (coils) Sheets Protein classification: Fibrous • Fibrous proteins are long and thin. They are the major structural materials for animal tissue; keratin, (hair, nails & feathers) collagen (muscles), fibroin (in silk). • Generally insoluble in water & resistant to acids & alkalis. Protein classification: Globular • Globular proteins have spiral chains folded into compact units. • Globular proteins are involved in maintenance and regulation processes due to their solubility in water. • Examples include all enzymes, many hormones, haemoglobin and antibodies. Enzymes • Enzyme function is related to the molecular shapes of proteins. • Denaturing of a protein involves physical alteration of the molecules as a result of temperature change or pH change. • The ease with which a protein is denatured is related to the susceptibility of enzymes to changes in temperature and pH. • Enzymes are most efficient within a narrow range of temperature and pH. Enzymes • Enzymes are all globular proteins. Enzymes catalyse specific reactions because of their specific active site. • Only molecules with the correct shape will fit onto the surface and it is only these molecules which will react using that particular enzyme as a catalyst. • When the enzyme is heated or the pH altered the shape of the molecule may change because the pattern of intermolecular bonding between chains is altered. • Chemists and biologists call this change to the enzyme denaturing since it can no longer carry out the specific reaction it was originally used for. Fats & oils Natural fats & oils • Depending on their origin can be classified as: Vegetable Animal Marine Fatty acids • Can be saturated or unsaturated straight chain carboxylic acids containing even numbers of carbon atoms. (C4-C24) • Fats and oils consist largely of mixtures of triglycerides in which the 3 fatty acids attached to the glycerol may be different. Hydrolysis of fats & oils • Fats & oils are esters. • When fats and oils are heated with superheated steam they break up or hydrolyse to form • Gylcerol • Fatty acids Ratio of 3 fatty acids to 1 glycerol Soaps • Soaps are produced by the alkaline hydrolysis of fats and oils. • This hydrolysis is usually carried out using sodium or potassium hydroxide. • Glycerol is liberated and used as a raw material in other processes. • The fatty acids are produced in the form of their potassium or sodium salts. These salts are soap! Soaps Hydrocarbon tail. Soluble in oils and grease. Ionic head is soluble in water. The hydrocarbon ‘tail’ bonds to greasy material in fabrics or on the skin. The ionic ‘head’ is attracted to the polar covalent water molecules. An emulsion of the grease in water is then formed which can be easily washed away. Test for unsaturation? • How do fats & oils effect bromine water? What's the difference? • Fats are solid at room temperature, oils are liquids. • The difference in melting point comes from the higher degree of unsaturation in oils. Van der Waals Fat Low level of unsaturation Van der Waals Oil Higher level of unsaturation Can we change an oil into a fat? • The conversion of an oil to a fat involves removal of unsaturation by addition of hydrogen. Nickel Hydrogenation Healthy diet? • Fats and oils are important in a balanced diet and supply the body with energy in a more concentrated form than carbohydrates. • There is evidence of a link between a high intake of saturated fat in the diet and heart disease. • There is further evidence to suggest that unsaturated fats may help to lower cholesterol.