EDTA
advertisement
Chapter 11
EDTA Titrations
Polar groups have positive and negative regions
that attract neighboring molecules by electrostatic
forces.
Nonpolar groups have little charge separation and
are soluble inside the nonpolar cell membrane.
Metal cations dissolve in water and are said to be
hydrophilic (“water loving”).
Cell membranes exclude water and are described as
hydrophobic (“water hating”).
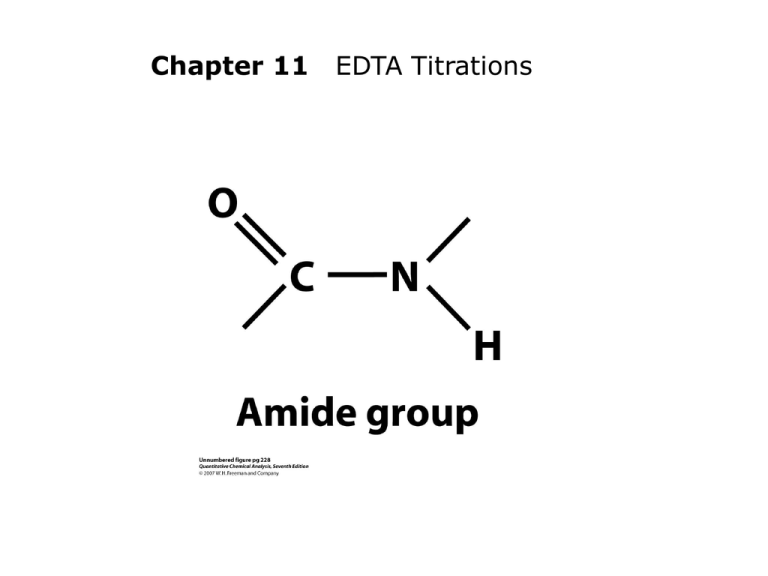
EDTA is a merciful abbreviation for ethylenediaminetetraacetic acid, a
compound that forms strong 1:1 complexes with most metal ions (Figure 121) and finds wide use in quantitative analysis.
11-1 Metal-Chelate Complexs
Metal ions are Lewis acids, accepting electron pairs from electron-donating
ligands that are Lewis bases.
Cyanide (CN-) is called a monodentate ligand because it binds to a metal
ion through only one atom (the carbon atom).
A ligand that attaches to a metal ion through more than one ligand atom is
said to be multidentate (“many toothed”) , or a chelating ligand (
pronounced KEE-late-ing).
We say that ethylenediamine is bidentate because it binds to the metal
through two ligand atoms.
The chelate effect is the ability of multidentate ligands to form more stable
metal complexes than those formed by similar monodentate ligands.4
An important tetradentate ligand is adenosine triphosphate (ATP), which
binds to divalent metal ions (such as Mg2+, Mn2+, Co2+, and Ni2+) through
four of their six coordination positions (Figure 12-2).
The octadentate ligand in Figure 12-3 is being evaluated as an anticancer
agent.5
The chelate is covalently attached to a monoclonal antibody, which is a
protein produced by one specific type of cell in response to one specific
foreign substance called an antigen.
A titration based on complex formation is called a complexometric titration.
The stoichiometry is 1:1 regardless of the charge on the ion.
BOX 11-1 Chelation Therapy and Thalassemia
11-2 EDTA
Acid-Base Properties
pK applies at 25oC and µ = 0.1, except pK1 applies
at µ = 1 M
Fraction of EDTA in the form Y4-:
where [EDTA] is the total concentration of all free EDTA species in the solution.
EDTA Complexes
The equilibrium constant for the reaction of a metal with a ligand is called the
formation constant, Kf, or the stability constant:
Formation constant:
Conditional Formation Constant
[Y4-] = αY [EDTA]
4-
Conditional formation constant:
The number K’f = αY4-Kf is called the conditional formation constant, or the
effective formation constant.
Mn+ + EDTA = MYn-4
K’f = αY4-Kf
11-3 EDTA Titration Curves
Mn+ + EDTA = MYn-4
K’f = αY4-Kf
Region 1: Before the Equivalence Point
Region 2: At the Equivalence Point
MYn-4 = Mn+ + EDTA
Region 3: After the Equivalence Point
(12-7)
Titration Calculations
Ca2+ + EDTA CaY2K’f = αY Kf = (0.30)(1010.65) = 1.34 X 1010
4-
Region 1: Before the Equivalence Point
Initial volume
of Ca2+
Fraction
remaining
(=4/5)
Original
Dilution
concentration factor
of Ca2+
Total volume
of solution
=0.029 1 M pCa2+ = -log[Ca2+] = 1.54
Region 2: At the Equivalence Point
Initial volume
of Ca2+
Original
Dilution
concentration factor
of Ca2+
Initial concentration (M)
Final concentration (M)
Total volume
of solution
Region 3: After the Equivalence Point
Volume of
excess EDTA
Original
Dilution
concentration factor
of EDTA
Total volume
of solution
Initial volume
of Ca2+
Original
Dilution
concentration factor
of Ca2+
Total volume
of solution
The Titration Curve
11-4 Do It with a Spreadsheet
Mass balance for M:
Mass balance for L:
Spreadsheet equation for
titration of M with L:
Spreadsheet equation for
titration of M with L:
11-5 Auxiliary Complexing Agents
To permit many metals to be titrated in
alkaline solutions with EDTA, we use an
auxiliary complexing agent.
Metal-Ligand Equilibria17
M + L = ML
β1 = [ML]/([M][L])
(12-13)
M + 2L = ML2
β2 = [ML2]/([M][L]2)
(12-14)
The equilibrium constants, βi, are called overall or cumulative formation
constants.
αM = [M]/CM
CM = [M] + [ML] + [ML2]
CM = [M] + β1[M][L] + β2[M][L]2
= [M]{1 + β1[L] + β2[L]2}
Fraction of free
metal ion:
(12-15)
EDTA Titration with an Auxiliary complexing Agents
K’’f = αZn αY Kf
2+
4-
(12-18)
Box 11-2 Metal Ion Hydrolysis Decreases the Effective Formation
Constant for EDTA Complexes
K’’’f = {(αFe αY )/αFeY }Kf
3+
4-
-
Take-home message: In this book,
we restrict ourselves to cases in
which there is no hydrolysis and
αMm+ is controlled by a
deliberately added auxiliary
ligand. In reality, hydrolysis of
Mm+ and MY influences most
EDTA titrations and makes the
theoretical analysis more
complicated than we pretend in
this chapter.
11-6 Metal Ion Indicators
Metal ion indicators (Table 12-3) are compounds whose color changes when
they bind to a metal ion. Useful indicators must bind metal less strongly than
EDTA does.
MgIn + EDTA MgEDTA + In
Red
Colorless
Colorless
Blue
(12-19)
Demonstration 11-1 Metal Ion Indicator Color Changes
If a metal does not freely dissociate
from an indicator, the metal is said
to block the indicator.
11-7 EDTA Titration Techniques
Direct Titration
In a direct titration, analyte is titrated with standard EDTA.
Auxiliary complexing agents such as NH3, tartrate, citrate, or triethanolamine
may be employed to prevent metal ion from precipitating in the absence of
EDTA.
Back Titration
In a back titration, a known excess of EDTA is added to the analyte.
Displacement Titration
Hg2+ does not have a satisfactory indicator, but a displacement titration is feasible.
Mn+ + MgY2- MYn-4 + Mg2+
(12-20)
2Ag+ + Ni(CN)42- 2Ag(CN)2- + Ni2+
Indirect Titration
Anions that precipitate with certain metal ions can be analyzed with EDTA by
indirect titration.
Box 11-3 Water Hardness
Hardness is the total concentration of alkaline earth (Group 2) ions, which are
mainly Ca2+ and Mg2+, in water.
Ca2+ + 2RCO2- Ca(RCO2)2(s)
(A)
Soap
Precipitate
R is a long-chain hydrocarbon such as C17H35ㅡ
Hard water leaves solid deposits called scale on pipes when it evaporates.
Hardness is beneficial in irrigation water because alkaline earth ions tend to
flocculate (cause to aggregate) colloidal particles in soil and thereby increase
the permeability of the soil to water.
CaCO3(s) + CO2 + H2O Ca(HCO3)2(aq)
(B)
The fraction of hardness due to Ca(HCO3)2(aq) is called temporary hardness
because this calcium is lost (by precipitation of CaCO3) upon heating. Hardness
arising from other salts (mainly dissolved CaSO4) is called permanent hardness
because it is not removed by heating.
Masking
A masking agent is a reagent that protects some component of the analyte from
reaction with EDTA.
When cyanide is added to a solution containing Cd2+ and Pb2+, only Pb2+ reacts
with EDTA.
(Caution: Cyanide forms toxic gaseous HCN below pH 11. Cyanide solutions
should be strongly basic and only handled in a hood.)
Fluoride masks Al3+, Fe3+, Ti4+, and Be2+.
(Caution: HF formed by F- in acidic solution is extremely hazardous and should
not contact skin and eyes. It may not be immediately painful, but the affected
area should be flooded with water and then treated with calcium gluconate gel
that you have on hand before the accidents. First aid providers must wear rubber
gloves to protect themselves.)
Demasking releases metal ion from a masking agent.