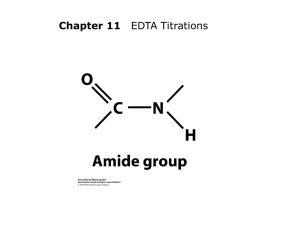
Int. ref. : C / 014 June `02
advertisement

EAC – European Aminocarboxylates Committee Int. ref. : C / 014 June ‘02 EDTA – FACTS ON ENVIRONMENTAL ISSUES The information presented here is based on the environmental section of the EU Risk Assessment Report (Draft of June 2001) that summarizes the present scientific knowledge on EDTA. This draft EU Risk Assessment Report was written by the German authorities -acting as Rapporteur- and approved in 2001 during the Environmental Sessions of the EU Technical Meetings on Existing Chemicals following Council Regulation 793/93/EEC. Biodegradation Laboratory tests The biodegradation rate of chelating agents in general depends strongly on the concentration of the chelating agent, the species of the complexed metal ions and the pH of the medium. In standard biodegradability tests EDTA is not readily biodegradable. However under alkaline conditions ready biodegradation of EDTA was observed. These test results could be relevant for the behavior in the environment as the pH of central European lakes and rivers ranges from 7.78.5. In tests with surface water at pH 6.5 the CaNa2-EDTA complex was biodegradable as well, however with longer lag times. Besides pH also counter ions exert an influence on biodegradability. The weaker Ca-, Mg- and Mn-EDTA complexes are biodegradable and the stronger complexes such as Cu- and Fe-EDTA are not biodegradable in laboratory tests. Wastewater treatment plants In standard industrial wastewater treatment plants EDTA removal rates up to 95% were obtained when facilities were operated at slightly alkaline conditions. In municipal wastewater treatment plants the optimal removal conditions (high EDTA concentrations and alkaline conditions) will normally not be present. Consequently in municipal wastewater treatment facilities little or no EDTA will be removed. Aqueous environment In view of the biodegradation mechanisms, OECD tests and results in full-scale wastewater treatment plants EDTA should be considered as inherently biodegradable. Due to biodegradation in combination with metal ion exchange reactions EDTA will not be persistent in the aqueous environment. Nevertheless for the purpose of the EU Risk Assessment the authorities use worstcase assumptions and EDTA is regarded to be non-biodegradable in surface waters. Soil EDTA is biodegraded in soil under aerobic conditions at an estimated half-life of 120-300 days. In soil all metal species degraded at the same rate with exemption of the Ni-EDTA complex that degrades more slowly. Sediment EDTA is degradable at aerobic conditions with a half-life of 200-300 days. In anaerobic parts of the sediment there are no signs of biodegradation after 7 weeks. Photochemical degradation The Fe (III) EDTA is photodegradable in aqueous solution. A laboratory test resulted in an estimated half-life of about 11 minutes. The half-life in river water in Central Europe was estimated at 5-480 hours depending on the extent of light penetration. Co (III) and Mn (II) complexes of EDTA were also found to be photodegradable, however at 10-20 times lower reaction rates. Chemistry making a world of difference European Chemical Industry Council Avenue E. van Nieuwenhuyse 4 B - 1160 Brussels Belgium Tel: +32 2 676 72 11 Fax: +32 2 676 73 01 mail@cefic.be www.cefic.org 2 Other metal complexes of EDTA are not photodegradable. About 50% of all discharged EDTA is estimated to be released as iron complex. Photodegradation of EDTA results in a number of biodegradable degradation products. Degradation products Degradation of EDTA results in a number of biodegradable degradation products. Some of these degradation products spontaneously transform into ketopiperazines by intermolecular cyclization. KPDA approximates readily biodegradability in standard biodegradation tests. Nevertheless KPDA was detected as the main degradation product in surface waters at µg/l ranges which can be explained by a steady state between its rapid formation from photodegradation processes and its removal by biodegradation. KPDA was demonstrated to be non-toxic to Fish, Daphnia and Algae (LC50 / EC50 > 100 mg/l). The EU Risk Assessment Report concludes that there is no risk for the aquatic environment from KPDA and further metabolites of EDTA. Bioaccumulation No accumulation in living organisms and via the food chain. Heavy Metals The EU risk assessment report concludes with the expectation of no risk for the aqueous environment due to the influence of EDTA on the mobility of heavy metals. This conclusion may seem to be in contrast to previously published results from model experiments where it has been demonstrated that EDTA can solubilize metals from sediments. Most of these studies however are not relevant to real environmental conditions primarily for two reasons; First, the test concentrations used were far too high; Second, EDTA is used in uncomplexed form while in the environment EDTA always will be present as metal- or Cacomplex. Solubilization The hypothesis that complexing agents such as EDTA would be able to raise the concentrations of heavy metals in the aquatic environment was discussed. After their application complexing agents will be present as metal - or calcium complexes. One EDTA molecule is able to bind and dissolve metal ions in an one to one equimolar ratio. In the aquatic environment there is a large excess of metal ions when compared to EDTA. Therefore EDTA can not significantly influence or raise the levels of heavy metals in the aquatic environment. The concentration of solubilized heavy metals is affected much more by natural causes -such as pH, precipitating anions and high molecular humic and fulvic acids- than by trace amounts of EDTA. In wastewater from a German municipal wastewater treatment plant and in surface water from a Spanish river it was demonstrated that the fraction of EDTA complexation from the total amount of complexed Cu, Zn and Cd metal ions was limited to a few percent only. Toxicity of heavy metals The influence of EDTA on the aquatic toxicity of heavy metals was investigated in a test on Daphnia. The toxicity of most metals decreased by a factor of 17 to 1700 with the exception of Hg, for which a different toxicity mechanism is assumed. Risk The EU Risk Assessment Report indicates that significant remobilization by EDTA can only occur in extreme cases where high concentrations of EDTA can be found, for example when large point sources are emitted into small rivers. In such extreme situations an EDTA induced increase in heavy metal concentrations can not be excluded. Eutrophication EDTA will only be able to stimulate growth of algae and higher plants in case of trace element deficiencies. Under normal conditions in the aqueous environment trace elements like Fe, Co, Mn and Zn will already be sufficiently available in soluble form. 3 Nutrient deficiency Under test conditions the addition of EDTA can induce deficiency of essential metal nutrients in the test medium resulting in a reduced algae growth. However these effects are not relevant for the aquatic environment where a stoichiometric surplus of metal nutrients is present. Aquatic toxicity In the Risk Assessment Report all relevant short-term and long-term ecotoxicity tests were evaluated. Valid long-term tests are available on three different species: Fish, Daphnia and Algae. Daphnia appeared to be the most sensitive species with a 21-d NOEC (long-term no effect concentration) of 25 mg/l of Na2H2-EDTA which is comparable to 22 mg/l of H4-EDTA. To account for the uncertainties involved a so-called ‘Assessment Factor’ is being applied that can vary from 10 to 1000 depending on the availability and quality of the toxicity data. For EDTA the PNECaqua (predicted no effect concentration in the aquatic environment) is determined by applying an assessment factor of 10 (related to the availability of three valid longterm tests for three different species) on the NOEC of the most sensitive species. The PNECaqua for EDTA is 2.2 mg/l. Extensive monitoring demonstrated that the concentration of EDTA in the aquatic environment is below this level. Nevertheless in some extreme situations close to highly concentrated large emission sources the presence of higher concentrations can not be excluded.





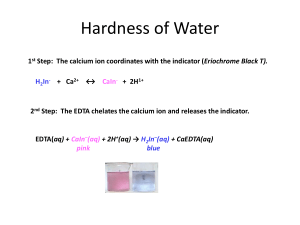
![[MIn - ]/[HIn 2](http://s2.studylib.net/store/data/005622090_1-a61ecc244fc4a127a11c4475c40f111e-300x300.png)