Complexometric Reactions (1)
advertisement

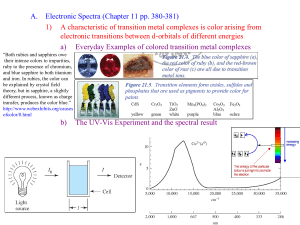
213 PHC 8th lecture (1) Gary D. Christian, Analytical Chemistry, 6th edition 1 • • • Define complexometric reactions. Differentiate between uni- and multidentate complexing agents. Understand the mechanism of complex formation and the effect of pH on it. 2 It is a reaction involves the formation of a substance called complex which is slightly ionising in solution. 3 Complex Formation Many metal ions form slightly dissociated complexes with various complexing agents. The no. of molecules of the complexing agent (ligand) depend on the coordination no. of the metal and on the no. of complexing gps. on the ligand. The complexes formed are stable. 4 Many cations will form complexes in solution with substances that have a pair of unshared electrons (complexing agents). The metal complexes formed with these complexing agents are 1:2, 1:4, 1:6, or 1:8 according to the coordination no. of the metal. There is only one complexing group on these complexing agents (unidentate). e.g.Ammonia (NH3) 5 Formation Constant (Kf) 2 ammonia molecules will complex with silver ion to form a colorless complex in a stepwise fashion. The equilibrium constant for each step, called the formation constant Kf : Ag+ + NH3 Ag(NH3)+ Kf1 = ……………… Ag(NH3)+ + NH3 Ag(NH3)2+ Kf2 = ……………… 6 The overall formation constant: Kf = Kf1 x Kf2 Kf = ………………… If the equilibrium is in the opposite direction, the constant is the reciprocal of the formation constant and is called the dissociation constant: Kd = 1 /Kf = ……………… 7 It is an organic agent that has two or more groups capable of complexing with a metal ion. The complex formed is called a chelate. The chelating agent is called the ligand. 8 The metal complexes formed with these complexing agents are often 1:1, regardless of the coordination no. of the metal ion. There are sufficient complexing groups on one chelating agent (multidentate). e.g.ethylenediaminetetraacetic acid (EDTA). 9 EDTA have four Ka values corresponding to the stepwise dissociation of the four protons: H4Y H+ + H3YH3Y- H+ + H2Y2H2Y2- H+ + HY3HY3- H+ + Y4- Ka1 = 1.0 x 10-2 Ka2 = 2.2 x 10-3 Ka3 = 6.9 x 10-7 Ka4 = 5.5 x 10-11 10 H4Y has a very low solubility in water, and so the disodium salt Na2H2Y2.2H2O is usually used, in which two of the acid groups are neutralized 11 The formation of the EDTA chelate with Ca2+: Ca2+ + Y4- CaY2- The formation constant: Kf = ……………… 12 The equilibrium in previous equation is shifted to the left as the H+ conc. (Why?). H+ conc. favor formation of the CaY4- chelate (Why?). The pH can affect also the metal ion. That is, OH- competes for the metal ion just as H+ competes for EDTA. 13 14 Definition of complexometric reactions. Types of complexing agents. Mechanism of complex formation. 15 16