“polyatomic” ion
advertisement

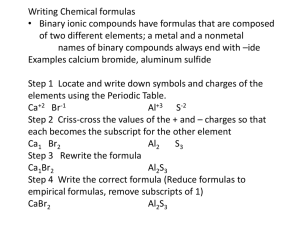
Chemical formula - combination of symbols that represent the composition of a compound Shows elements present and number of atoms subscripts Represent the number of atoms of that element in the compound No subscript is an “understood” 1 NaCl 1 Na 1 Cl H2SO4 2H 1S 4O Ca(ClO3)2 ????? 1 Ca 2 Cl 6O Two types of compounds we will learn how to write formulas for Ionic – transfer of electrons Covalent – share electrons (called Molecular) Molecular Compounds (covalent) unit – “molecule” (bonded covalently) Representative of elements – nonmetals Physical state – solid, liquid, gas Solids – low melting point, brittle Type – electrically neutral group of atoms that act as a unit Molecule Naming binary molecular compounds 2 elements in the compound Both nonmetals! 2 naming systems Prefix system Stock system (roman numerals) Both systems are correct I’m sure you will prefer the prefix system! PREFIXES YOU MUST MEMORIZE! Number of atoms 1 2 3 4 5 6 7 8 9 10 Prefix used mono di tri tetra penta hexa hepta octa nona deca When 2 nonmetallic elements combine Often do so in more than one way Example CO CO2 Problem with calling them both “carbon oxide” CO2 – you exhale. It is normally present in the air you breathe CO – hopefully is not in the air you breathe In large amount R.I.P. Catalytic converter – cars Converts CO to CO2 Prefix + first element name Followed by prefix + 2nd element name with “ide” ending ******only time you can not use a prefix is if the first element in the compound is a single atom PCl3 phosphorus trichloride CO Carbon monoxide (not monocarbon monoxide!) Don’t use “double vowels” Change if a “tongue twister” Monooxide monoxide Decaoxide decoxide Trioxide – is fine N2O Dinitrogen monoxide SF6 Sulfur hexafluoride N2H4 Dinitrogen tetrahydride NO FACTORING ALLOWED!!! P2O3 Diphosphorus trioxide Name the following: • CS2 • Carbon disulfide • Cl2O7 • Dichlorine heptoxide • P4O10 • Tetraphosphorus decoxide • N2O5 • Dinitrogen pentoxide • CCl4 • Carbon tetrachloride Write formulas for the following: •carbon tetrabromide •CBr4 •dinitrogen tetrahydride •N2H4 • boron trichloride • BCl3 • diphosphorus trioxide • P2O3 •A molecular compounds worksheet just for you!! IONIC COMPOUNDS • Four different types we will learn about • Metal + nonmetal (binary ionic) • Metal + polyatomic ion • Polyatomic ion + polyatomic ion • Polyatomic ion + nonmetal Ionic Charges • Monatomic ions – ions consisting of only one atom • Charges can often be determined by using the periodic table • Metallic elements – tend to lose electrons to form cations • Group 1 – all 1+ • Group 2 – all 2+ Nonmetals • Nonmetals tend to gain electrons when they bond with metals – form anions Transition metals • Many have more than one common ionic charge Are going to use roman numerals I, II, III, IV, V Oxidation number • Indication of how many electrons it will gain or lose when it forms a bond • Gains or loses electrons – forms an ion • Charged particle • Can be found for each element on the periodic table • Refer to yours!!! Some elements have more than 1 oxidation number – that means they can form more than one type of compound When a single atom takes on a charge (by gaining or losing electrons) – it forms a “monatomic ion” Ion made up of more than 1 atom – “polyatomic” ion • Monatomic ions • Na+ Ca+2 Cl- O-2 • Polyatomic ions • CO3-2 ClO3- OH- Why would an atom want to form an ion? Remember the “octet rule” Wants a filled outer shell For most atoms, that is 8 Samples on board using electron dot notation Na and Cl Ca and Cl Al and Cl Ca and S K and N you do Metal always written first – has positive oxidation number (written first) Nonmetal written second – has negative oxidation number Can use “criss-cross” method to arrive at correct formula. Must remember to factor subscripts if possible!! Magnesium oxide - MgO Metal full name first Nonmetal name with “ide” ending NaCl sodium chloride CaCl2 calcium chloride LiF lithium fluoride AlBr3 aluminum bromide Use Roman numerals to specify the oxidation number used I, II, III, IV, V, VI, VII, VIII Transition metals characteristically have multiple oxidation numbers ONLY USE ROMAN NUMERALS IF THE METAL HAS MORE THAN ONE OXIDATION NUMBER LISTED Nonmetals may have more than one oxidation number, you just use the first number listed NEGATIVE Co, Ni, Cu, Fe, Mn Always check before writing name for the compound FeCl2 Iron(II) chloride FeO Fe2O3 calcium sulfide CaS strontium bromide SrBr2 chromium(III) chloride CrCl3 iron(II) oxide FeO Fe2O3 Iron(III) oxide KI Potassium iodide CuO Copper(II) oxide NiCl3 Nickel(III) chloride CrO3 Chromium(VI) oxide 2. Metal + polyatomic ion Almost all polyatomic ions have a negative charge 2 you are responsible for have a positive charge NH4+ and H3O+ Polyatomic ions travel as a unit Page 102 in text - list of polyatomic ions You will use the table I gave you NEVER CHANGE THE SUBSCRIPTS IN A POLYATOMIC ION THAT MEANS NEVER!!!!!!! Can use same “criss-cross” method for determining the correct formula Same rules apply – must factor the subscripts if you can (only the oxidation numbers that are used – NOT THE SUBSCRIPTS OF THE POLYATOMIC ION!!!!!! At first, always put a parenthesis around the polyatomic ion Only time the parenthesis can be dropped is if a “1” criss-crosses down or if the subscript factors to a “1” Don’t forget to include a roman numeral in the name if the metal has multiple oxidation numbers!!!!!! Metal name first (only use roman numeral if the metal has more than one oxidation number!!!) Second is the name of the polyatomic ion – taken directly from the table!! Don’t make up your own name!!!!!!!! Representative unit = “formula unit” Type of elements: Metal with nonmetal Physical state: crystalline solid (hard) High melting point Most are soluble in H2O Poor conductors of electricity in the solid state But good conductors when melted (molten) or dissolved in H2O (aq) (ions free to move) Oxyacids – contain H, O and a third element (usually a nonmetal) Acetic HCH3COO (acetate ion) Nitric HNO3 (nitrate ion) Nitrous HNO2 (nitrite ion) Phosphoric H3PO4 (phosphate ion) Sulfuric H2SO4 Sulfurous H2SO3 Carbonic H2CO3 Hypochlorous HClO Chlorous HClO2 Chloric HClO3 Perchloric HClO4 (sulfate ion) (sulfite ion) (carbonate ion) (hypochlorite ion) (chlorite ion) (chlorate ion) (perchlorate ion) HF HCl HBr HI hydrofluoric acid hydrochloric acid hydrobromic acid hydroiodic acid