Wed Feb 27 Naming & Formulas Part 3 PowerPoint
advertisement

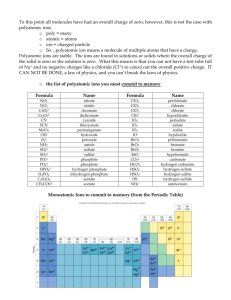
WRITING NAMES & FORMULAS FOR POLYATOMIC IONS & RELATED OXYACIDS Textbook Pages: 66 – 67, 69 - 70 Polyatomic Ions • Definition: A polyatomic ion is a group of atom that stay together and have an overall ionic charge. • Example: NO3− Nitrate ion • ***Refer to the back of your Periodic Table for a list of the Polyatomic Ions!!! The “Standard” Polyatomic Ions • There is a special way to name these ion. The following are the most common forms of the ions: • The Standards: • ClO3 • BrO3 – • IO3 • NO3 • SO4 2− • CO3 2− • PO4 3− Polyatomic Ions: chlorate ion bromate ion iodate ion nitrate ion sulphate ion carbonate ion phosphate ion ***All “standard” polyatomic ions end in “ate” Altering the Number of Oxygens in Polyatomics • 1. If there is one more Oxygen than the standard ion, the ion follows the following pattern: per ________ ate • Example: ClO4− perchlorate • Note: the charge does not change, it stays the same as the standard/original polyatomic ion. Altering the Number of Oxygens in Polyatomics • If there is one less Oxygen then the standard ion, the ion follows the following pattern: ________ ite • Example: ClO2− chlorite • Note: the charge does not change, it stays the same as the standard/original polyatomic ion. Altering the Number of Oxygens in Polyatomics • 3. If there are two less Oxygen than the standard ion, then the ion follows the following pattern: hypo ________ ite • Example: ClO− hypochlorite • Note: the charge does not change, it stays the same as the standard/original polyatomic ion. Oxyacids • Definition: Oxyacid is an acid composed of hydrogen, oxygen, and atoms of at least one other element. • Oxyacids follow the pattern: ic acid • Example: HClO3 Chloric Acid Oxyacids • There is a special way to name these acids. • The Standards: • HClO3 • HBrO3 • HIO3 • HNO3 • H2SO4 • H2CO3 • H3PO4 Oxyacids (Add H+): chloric acid bromic acid iodic acid nitric acid sulphuric acid carbonic acid phosphoric acid ***All “standard” oxyacids take the format : ______ ic acid Altering the Number of Oxygens in Oxyacids • 1. If there is one more Oxygen that the standard, the acid follows the following pattern: per ________ ic acid • Example: HClO4 perchloric acid Altering the Number of Oxygens in Oxyacids • 2. If there is one less Oxygen that the standard, the acid follows the following pattern: ________ ous acid • Example: HClO2 chlorous acid Altering the Number of Oxygens in Oxyacids • If there are two less Oxygen than the standard, the acid follows the following pattern: hypo ________ ous acid • Example: HClO hypochlorous acid *** Remember: PRACTICE MAKES PERFECT!!!! SO PRACTICE A LOT!!!!!!!!!!