Conductivity2
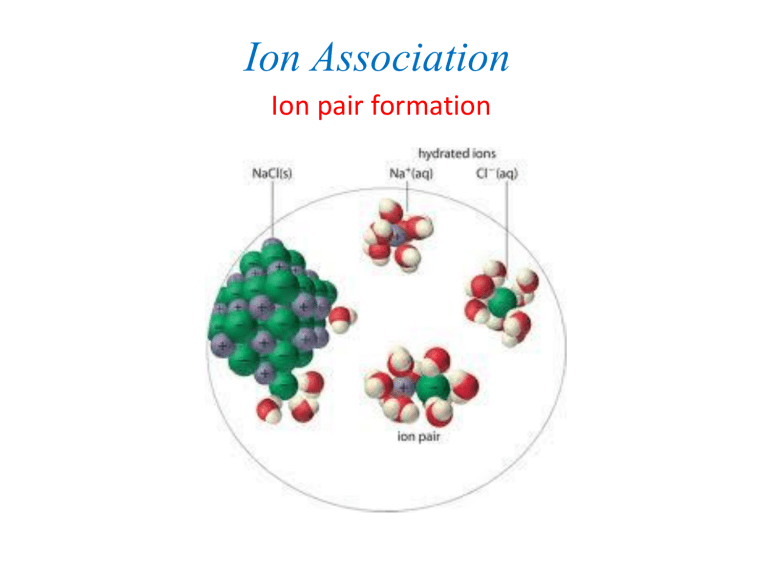
Ion Association
Ion pair formation
Ion Association
Ion pair formation fully solvated solvent-shared or solventseparated
Ion pair contact ion pair
Ion pairs formed when opposite ions come close enough to be separated by a distance < r*
Bjerrum
As
Charge of ions increases (z i
): r* increases, probability of ion pair formation increases.
Temperature increases , r* decreases, probability of IP formation decreases. Kinetic energy acts against attraction.
Polarity of solvent increases ( e r
), attraction decreases, probability of IP formation decreases
Electrostatic potential energy for interaction between two univalent ions:
Substitute r = r*
For univalent electrolyte in water at 25 o C, IP formation is negligible r* is only 0.358 nm.
Very difficult to bring the opposite ions so close!
Ionic
Mobilities
Transport Numbers
c = 1 M c
+
= a M c
-
= b M
Electroneutrality condition
c = 1 M c
+
= a M c
-
= b M
Let 4F be passed through the cell; t
+
=3t
-
Before electrolysis
On electrolysis
After electrolysis
Let 4F be passed through the cell; t
+
=3t
-
4 Cl -4e
2 Cl
2
3 mol H +
1 mol Cl -
4 H + +4e -
2 H
2
3 mol H +
1 mol Cl
For anodic region:
C residual
= C initial
– C react
+ C transfer
3 = 6 – 4 + C transfer t
-
= 1 / 4 = 0.25
t
+
= 3 / 4 = 0.75
measurement of transport numbers by Hittorf method
The method of
Hittorf is based on concentration changes in the anodic region and cathodic region in an electrolytic cell, caused by the passage of current through the electrolyte.
Let 1F be passed through the cell;
t
amount lost in cathode compartmen t total amount lost t
amount lost in anode compartmen t total amount lost
A solution of LiCl was electrolyzed in a Hittorf cell. After a current of
0.79 A had been passed for 2 h, the mass of LiCl in the anode compartment has decreased by 0.793 g. a. Calculate the transport numbers of Li + and Cl .
b. If L o
(LiCl) is 115 W -1 cm 2 mol -1 , what are the molar ionic conductivities and the ionic mobilities?
2) The moving-boundary method
MA, MA’ have an ion in common. The boundary, rather difference in color, refractivity, etc. is sharp.
In the steady state, the two ions move with the same velocity.
When Q coulomb passes, the boundary moves x, the cross-sectional area of the tube is A:
No. of mole of H+ that passed from AA’ to BB’ n=c.V=c
+
.A.x
Charge carried by these moles:
Q
+
=z
+
F n
+
=z
+
F c
+
A x t
Q
Q
z
I
F
c
t
A x
Sample:
When A = 1.05
× 10 -5 m 2 , c(HCl) = 10.0 mol m -3 ,
I = 0.01 A for 200 s, x was measured to be 0.17 m.
Calculate t (H + ).
Solution: t
+
= 0.17 m × 1.05 × 10 -5 m 2 × 10.0 mol m -3 × 1
× 96500 C mol -1 / 0.01 A × 200 S
= 0.82
Kohlrausch’s Law of
Independent migration
Valid only at infinite dilution!
Experimentally determined
L o
CH
3
COOH
L o
HCl
L o
CH
3
COONa
L o
NaCl
-
-
L o
CH
3
COOH
L o
HCl
L o
CH
3
COONa
L o
NaCl
Grotthuss Mechanism
Explains the high conductivity of H + and OH in water
Ion Solvation
Size
But larger size means slower motion, the conductivity should drop?!!!!
•
Smaller size,
• larger interaction with water molecule,
• hydrate shell larger on Li + ,
• moving species larger,
•
Lower conductivity
/ mPas
K +
Viscosity of the solvent
acetone
0.316
Methyl alcohol
0.547
Ethyl alcohol
1.200
0.0082
0.0054
0.0022
Li + 0.0075
0.0040
0.0015
Walden’s Rule
L.











