ppt
advertisement

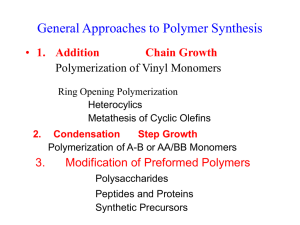
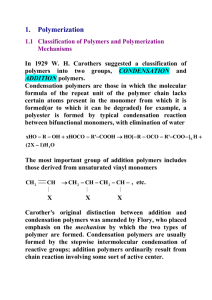
Polymers Basic Definitions • Polymer: Any long-chain molecule synthesized by linking together single parts called monomers. • Monomer: The simplest nonredundant unit from which a polymer is synthesized. • Elasticity: The property of a material to return to its original shape when stretched. • Plasiticity: The property of a material to stay deformed with stretched. • Plastic: A material that exhibits plasticity or a colloquial term for a polymer. Synthetic Polymers Synthetic polymers (that is, not biopolymers like proteins and DNA) can be divided into two main categories: A. Addition polymers, a.k.a. chain-growth polymers: • Vinyl monomers bond together by splitting or moving pi bond. • Polymerization proceeds through a reactive intermediate as a free radical, carbocation or carbanion. • Examples are poly(vinyl chloride), polyethylene, polystyrene. B. Condensation polymers, a.k.a. step-growth polymers • Functional groups on the ends of monomers react to form new bonds and thus create a polymer. • Common by-products of condensation polymerization are H2O or HCl. • Reactions are often nucleophilic acyl substitution reactions between monomers that are carboxylic acid derivatives. • Examples include polyester, Nylon, polycarbonate. Drawing Polymer Structures • Show the structure by placing parentheses around the repeat unit. • n = average degree of polymerization • Note that the chain doesn’t have any extra carbons, just extra covalent bonds (the black bonds above). Nomenclature • To name a polymer, prefix poly to the name of the monomer from which the polymer is derived. • If the name of the monomer is one word, no parentheses are necessary. • For more complex monomers or where the name of the monomer is two words, enclose the name of the monomer in parentheses, as for example poly(vinyl chloride). Morphology • The architecture of polymers is quite varied. Some have linear and branched chains. Others have comb, ladder, and star structures. • Most commercial polymers are linear or lightly branched chains. • Some polymers such as rubber and Bakelite are cross-linked which gives polymers added strength. • Polymers in the solid state tend to have both ordered crystalline domains and disordered amorphous domains. • Some polymers are cooled or precipitated, tend to partially crystallize. • Polymers with complicated and irregular shapes, which prevent efficient packing into ordered structures, are usually in the amorphous state. http://www2.dupont.com/Plastics/en_US/Products/ Zytel_HTN/Zytel_HTN_whitepaper_R8.html • Polymers with crystalline regions have either: 1. Regular, compact structures so that a chain can fold back upon itself and be stuck together with dispersion forces. 2. Polar functional groups so that hydrogen bonding can bind regions in the same polymer chain or among different chains. • As the degree of crystallinity increases, the polymer becomes more opaque due to scattering of light by the crystalline domains. • Contrary to popular perception, transparent materials are amorphous, not crystalline. • Melt transition temperature, Tm: The temperature at which crystalline regions melt • as the degree of crystallinity increases, Tm increases Thermal Properties of Polymers • Thermoplastic: A polymer that can be melted and molded into a shape that is retained when it is cooled. • Thermoset: A polymer that can be molded when first prepared, but once it is cooled, hardens irreversibly and cannot be remelted. • Highly amorphous polymers, on heating, are transformed from a hard glass to a soft, flexible, rubbery state. • Glass transition temperature, Tg: The temperature at which a polymer undergoes a transition from a hard glass to a rubbery solid Step-Growth Polymers • Step-growth polymerization: a polymerization in which chain growth occurs in a stepwise manner when a functional group from one monomer reacts with a functional group of another monomer. • Monomers are usually difunctional. • Chain propagation occurs in both directions. • We’ll examine five types of step-growth polymers. • • • • • Polyamides Polyesters Polycarbonates Polyurethanes Epoxy resins Polyamides • While there are many synthetic routes to make polyamides (nylons), the reaction of a diol with a diamine is common. • Hexanedioic acid reacts with 1,6-hexanediamine to make Nylon 6,6. • The raw material base for the production of Nylon 66 is benzene, which is derived from cracking and reforming of petroleum. • Catalytic reduction of benzene followed by air oxidation gives a mixture of cyclohexanol and cyclohexanone. • Oxidation of the mixture by nitric acid gives adipic acid. • Adipic acid is in turn used for the synthesis of 1,6-hexanediamine. • Nylon 6,6 is used in electrical insulation, carpet fibres, clothing, airbags, zip ties, ropes, conveyor belts, hoses, among other things. Nylon 6 • Nylon 6 is synthesized from a six-carbon monomer in a process called ring-opening polymerization. • Nylon 6 is fabricated into fibers, brush bristles, high-impact moldings, and tire cords. Kevlar • Kevlar is an aromatic polyamide (an aramid). • Cables of Kevlar are as strong as cables of steel, but only about 20% the weight. • Kevlar fabric is used for bulletproof vests, jackets, and raincoats, snowboards, drumheads, et. al. • Much of the strength of Kevlar originates from the hydrogen bonding that occurs between chains as well as the phenylene groups that make the individual chains more rigid. Polyesters • Poly(ethylene terephthalate), abbreviated PET or PETE, is fabricated into Dacron fibers, Mylar films, and plastic beverage containers. The key step in formation of this polymer is transesterification. • Ethylene glycol is synthesized from ethylene. • Terephthalic acid is synthesized from pxylene, which is obtained from petroleum refining. H2CrO4 • Poly(ethylene terephthalate) (PET) can be made with % crystalline domains ranging from 0% to 55%. n • Completely amorphous PET is formed by cooling the melt quickly. • PET with a low degree of crystallinity is used for plastic beverage bottles. • By prolonging cooling time, more molecular diffusion occurs and crystalline domains form as the chains become more ordered. • PET with a high degree of crystallinity can be drawn into textile fibers and tire cords. Polycarbonates • Polycarbonates, the most familiar of which is Lexan, are important engineering plastics. • Lexan is made from the disodium salt of bisphenol A (BPA) and phosgene. Phosgene is a diacid chloride. Polycarbonates • Polycarbonates, the most familiar of which is Lexan, are important engineering plastics – Lexan is made from the disodium salt of bisphenol A and phosgene O CH 3 + Na - O O- Na + CH 3 Di so diu m s a lt o f B is ph e no l A + Cl Cl Ph o sg e n e O CH 3 O O CH 3 Lex a n ( a pol y ca rbon a t e) + 2 NaCl n Bisphenol A • Lexan is a tough transparent polymer with high impact and tensile strengths and retains its shape over a wide temperature range. • It is used in sporting equipment, such as bicycle, football, and snowmobile helmets as well as hockey and baseball catcher’s masks. • It is also used in the manufacture of safety lenses and unbreakable windows. Polyurethanes • A urethane, or carbamate, is an ester of carbamic acid, H2NCH2COOH. • They are most commonly prepared by treating an isocyanate with an alcohol. • Polyurethanes consist of • flexible polyester or polyether units (blocks) alternating with urethane units (blocks) derived from a diisocyanate. • Polyurethane fibers are fairly soft and elastic and are used in “stretch” fabrics such as spandex and Lycra. Epoxy Resins • Epoxy resins are prepared by a polymerization in which one monomer contains at least two epoxy groups. • Epoxy resins are produced in forms ranging from low viscosity liquids to high melting solids. • The most widely used epoxide monomer is the diepoxide prepared by treating 1 mol of bisphenol A with 2 mol of epichlorohydrin. • Formation of a diepoxide by an SN2 mechanism. • The diepoxide monomer is then treated with a diamine that opens the highly strained epoxide ring by an SN2 mechanism. Chain-Growth Polymers • Chain-growth polymerization: A polymerization in which monomer units are joined together without loss of atoms. • From the perspective of the chemical industry, chaingrowth polymerization is the single most important reaction of alkenes. • Many commercially available polymers are produced from vinyl monomers using chain polymerization. Free Radical Polymerization • Free Radical: A molecule or ion containing one or more unpaired electrons. • To account for the polymerization of alkenes in the presence of peroxides, chemists propose a three-step radical chain mechanism. (1) chain initiation (2) chain propagation (3) chain termination • Among the initiators used for radical chaingrowth polymerization are organic peroxides, which decompose as shown on mild heating. • Fishhook arrow: A curved and barbed (fishhook) arrow used to show the repositioning of a single electron. • Step 1: Chain initiation • A step in a radical chain reaction characterized by the formation of radicals from nonradical compounds. • Step 2: Chain propagation • Chain propagation: a step in a radical chain reaction characterized by the reaction of a radical and a molecule to give a new radical. • Chain length, n: the number of times the cycle of chain propagation steps repeats in a chain reaction. • Chain propagation (cont’d) • Step 3: Chain termination • A step in a radical chain mechanism that involves destruction of radicals. • One type of chain termination is the combination of two free radicals. • Chain termination can occur via disproportionation where two free radicals meet, but rather than combine, an electron moves from one chain to another. • The stability of free radicals is similar to the stability of carbocations. (methyl < 1< 2< 3) • Free radical reactions with vinyl groups almost always give the more stable (the more substituted) radical. • Therefore, polymerizations of vinyl monomers tend to yield polymers with head-to-tail linkages. • The first commercial polyethylene produced by freeradical polymerization was the soft, tough polymer known as low-density polyethylene (LDPE). • LDPE chains are highly branched. • Because this branching prevents polyethylene chains from packing efficiently, LDPE is largely amorphous and transparent. • Approximately 65% is fabricated into films for consumer items such as baked goods, vegetables and other produce, and trash bags. • High-density polyethylene (HDPE) has fewer branches and is more crystalline than (LDPE); therefore, it is a stronger material. • It’s most common application is to make milk jugs. Recycling Codes • Many thermoplastic polymers can be recycled easily. • Polymers are commonly sorted according to a recycling code developed by the Society of the Plastics Industry in 1988. • Codes for the sorting of other materials have also been generated by other industry groups. • In Omaha, PETE (1), HDPE (2), PVC (3) and polypropylene (5) are recycled via trash pick-up. • Includes: water bottles, pop bottles, cooking oil bottles, mouthwash bottles, shampoo bottles, cleaning product bottles, milk jugs, juice bottles, margarine tubs, whipped topping tubs, yogurt containers, sour cream containers, reusable/disposable food storage containers, clamshell deli containers, DVD cases, microwave meal trays