Chapter 6. The Crystalline State
advertisement

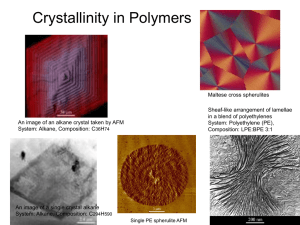
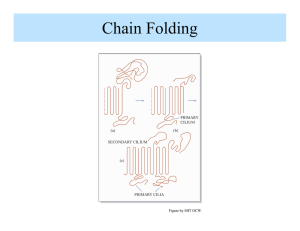
Chapter 6. The Crystalline State 6.1 General Considerations 6.1.1 Historical Aspects Staudinger Herman Mark Ziegler and Natta Macromolecular Hypothesis Father of Polymer Science stereospecific polymers 6.1.2 Melting Phenomena 6.1.3 Example Calculation of Percent Crystallinity 6.2 Methods of Determining Crystal Structure 6.2.1 A Review of Crystal Structure 6.2.2 X-Ray Methods 6.2.3 Electron Diffraction of Single Crystals 6.2.4 Infrared Absorption 6.2.5 Raman Spectra 6.3 The Unit Cell of Crystalline Polymers 6.3.1 Polyethylene 6.3.2 Other Polyolefin Polymers 31 72 41 6.3.3 Polar Polymers and Hydrogen Bonding 6.3.4 Polymorphic Forms of Cellulose Four different polymorphic forms of crystalline cellulose exist. 6.3.5 Principles of Crystal Structure Determination Experiments do not yield the crystal structure; only researchers’ imagination and hard work yield that. Natta and Corradini postulated three principles for the determination of crystal structures: 1. The equivalence postulate. 2. The minimum energy postulate. 3. The packing postulate. 6.4 Structure of Crystalline Polymers 6.4.1 The Fringed Micelle Model According to the fringed micelle model, the crystallites are about 10 nm long. The chains are long enough to pass several crystallites, binding them together. amorphous crystalline 6.4.2 Polymer Single Crystals 1957 Keller prepared single crystals of PE. 6.4.2.1 The Folded-Chain Model Adjacent reentry 10~20 nm thick (contour length ~ 200 nm) If the polymer solution is slightly more concentrated, or if the crystallization rate is increased, the polymers will crystallize in the form of various twins, spirals, and dendritic structures, which are multilayered. PEO-b-PS : the crystals reject the amorphous portion (PS), which appears on the surfaces of the crystals. 6.4.2.2 The Switchboard Model In the switchboard model the chains do not have a reentry into the lamellae by regular folding; they rather reenter more or less randomly. 6.5 Crystallization From the Melt 6.5.1 Spherulitic Morphology Maltese cross When the spherulites are nucleated simultaneously, the boundaries between them are straight. However, when the spherulites have been nucleated at different times, their boundaries form hyperbolas. Spherulites are composed of individual lamellar crystalline plates. Small-angle light scattering max is the angle at which the intensity maximum occurs is the wavelength U is radial direction R is the radius of the spherulite 6.5.2 Mechanism of Spherulite Formation 6.5.3 Spherulites in Polymer Blends and Block Copolymers 6.5.4 Percent Crystallinity in Polymers Where Ac and Aa represent the area under the Bragg diffraction line. 6.6 Kinetics of Crystallization 6.6.1 Experimental Observations of Crystallization Kinetics 6.6.2 Theories of Crystallization Kinetics 6.6.2.1 The Avrami Equation 6.6.2.2 Keith-Padden Kinetics of Spherulitic Crystallization d = D/G Where D is the diffusion coefficient for impurity in the melt and G reprents the radial growth rate of a spherulite. The quantity d, whose dimension is that of length, determines the lateral dimensions of the lamellae. Thus d is a measure of the internal structure of the spherulite, or its coarseness. Where DF* is the free energy of formation of a surface nucleus of critical size, DE is the free energy of activation for a chain crossing the barrier to the crystal 6.6.2.3 Hoffman’s Nucleation Theory 6.6.2.4 Example Clculation of the Fold Surface Free Energy 6.6.2.5 Three Regimes of Crystallization Kinetics 6.7 The Reentry Problem in Lamellae 6.7.1 Infrared Spectroscopy 6.7.2 Carbon-13 NMR 6.7.3 Smal-Angle Neutron Scattering 6.7.3.1 Single-Crystal Studies 6.7.3.2 Melt-Crystallized Polymers 6.7.4 Extended Chain Crystals 6.8 Thermodynamics of Fusion 6.8.1 Theory of Melting Point Depression 6.8.2 Example Calculation of Melting Point Depression 6.8.3 Experimental Thermodynamic Parameters 6.8.4 Entropy of Melting 6.8.5 The Hoffman-Weeks Equilibrium Melting Temperature 6.9 Effect of Chemical Structure on the Melting Temperature 6.10 Fiber Formation and Structure 6.10.1 X-Ray Fiber Diagrams 6.10.2 Natural Fibers 6.11 The Hierarchical Structure of Polymeric Materials 6.12 How Do You Know It’s a Polymer?