Chapter 15 Multistep Syntheses
advertisement

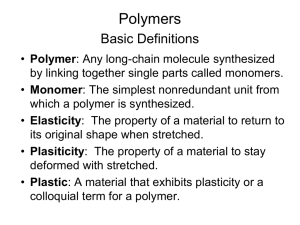
Chapter 16 Polymeric Materials • • • • • • • Monomers and Polymers Linear and Branched Polymers Types of Polymerization Addition Polymerization Condensation Polymers Extensively Cross-Linked Polymers (SKIP) Three-Dimensional Structure Polymers (SKIP p. 813-818 top) Chapter 16 Polymeric Materials • Polymer Nomenclature - Monomers and Polymers – Definitions of polymer, monomer, plastic, thermoplastic and thermoset plastic in “handout” – repeat units, average degree of polymerization • Polymer Architecture - Linear and Branched Polymers – See handout for depiction of linear, branched, star, comb, ladder and cross-linked networks • Polymer Morphology – ordered = crystalline regions – random = amorphous regions • Polymer properties and transitions – Modulus (degree of hardness/softness) – Glass Transition Temperature (transition from glassy to rubbery) – Melt Temperature(transition to viscous flow) Chapter 16 Polymeric Materials • Types of Polymerization • Addition (also called chain-growth) Radical (three steps – initiation, propagation, termination) Cationic Anionic (called “ living” polymer, can make block co-polymers) • Condensation (also called step-growth) loss of water – Polyester Polyamide Polycarbonate Polyurethane Epoxy resins Polysaccharides (starch) Polypeptides and proteins Chapter 16 Polymeric Materials • Addition Polymerization – Radical Polymerization • 3 steps (initiation, propagation, and termination) see handout – Ionic Polymerization • cationic • anionic (living polymers, useful to make “block” co-polymers) – Cross-Linking in Polymers (can use bi-functional, tri functional, quadri-functional monomers) – Heteroatom-Containing Addition Polymers • Polyols – can not make polyvinyl alcohol from vinyl alcohol, instead polymerize vinyl acetate and then hydrolyze to polyvinylalcohol • Polyethers (e.g., poly(ethylene glycol) • Polyacetals (e.g., paraformaldehyde from formaldehyde) Chapter 16 Polymeric Materials • Condensation Polymers – Polyesters (from a dicarboxylic acid and diol) – Polysaccharides (from carbohydrate (or sugar) condensation) – Polyamides (from dicarboxylic acid and diamine) – Polypeptides (from20 common amino acids) – Polyurethanes (form bis-isocyanate and diol) Chapter 16 Polymeric Materials • Three-Dimensional Structure of Polymers – Polypropylene (SKIP pages 813-818 top) – Naturally Occurring Polypeptides • Primary structure - amino acid sequence • Secondary structure – -pleated - deviation from planarity due to steric interactions – -helix - hydrogen bonding from peptide units in the same chain • Tertiary structure - spatially dispersed -pleated and -helix • Quaternary structure - several polypeptides form a complex – Cellulose and Starch • Starch from -glucose (axial C1-OH group) • Cellulose from -glucose (equatorial C1-OH group) Chapter 16 Polymeric Materials • Review of Reactions – Be able to draw the basic polymerization mechanism for • radical, anionic, cationic • general condensation polymerization for a polyester, polyamide, polyurethane, polypeptide • Review Problems – Especially review problem 16.1 – Know nomenclature of synthetic and natural polymers – Know how to identify repeat units Chapter 16 Polymeric Materials • Summary – Definitions for polymer, monomer, plastic, thermoplastic, thermoset, melt temperature, glass transition temp. – Nomenclature for simple addition and condensation polymers – Linkages between monomers include • C-C bonds in vinyl polymers • C-O bonds in polyesters, polyacetals, polyethers, polysaccharides • C-N bonds in polyamides, polypeptides – Role of Hydrogen bonding in nitrogen and oxygen containing polymers • intermolecular H-bonding in pleated peptides • intramolecular H-bonding in helix peptide structures • primary, secondary, tertiary and quaternary structures