The Structure and Properties of Polymers
advertisement

The Structure and Properties of
Polymers
By:Sawsan
D.Shubbar
V
What is a polymer?
• A long molecule made
up from lots of small
molecules called
• monomers.
All the same monomer
• Monomers all same
type (A)
• A+A+A+A
• -A-A-A-A• eg poly(ethene)
polychloroethene
PVC
Different monomers
• Monomers of two
different types A + B
• A+B+A+B
• -A-B-A-B• eg polyamides
• polyesters
Copolymers
• Copolymers are like polymer alloys.
Different mers are joined to form a mixture
in the backbone, eg. ABS.
• they can be tailored to obtain specific
properties.
Thermoplastics (80%)
• No cross links between chains.
• Can change shape.
• Can be remoulded.
Thermosets
• Cross-linking formed by covalent
bonds.
• Bonds prevent chains moving relative
to each other.
What Makes Polymers Unique?
• Really big molecules (macromolecules) like
polymers have very different properties than
small molecules. When polymer is melted,
the chains can flow past each other.
Chain entanglement: Long polymer chains
get entangled with each other.
Molecular Weight of Polymers
Unlike small molecules, polymers
are typically a mixture of differently
size molecules.
Mv Mn
Only an average molecular
Mw
weight can be defined.
#
o
f
m
o
le
cu
le
s
•
•
•
•
increasing molecular weight
Longer chains make stronger
polymers.
• There is a critical length
needed before strength
increases.
• An average No. of 100
repeating units is
necessary for HC
polymers but only 40 for
nylons.
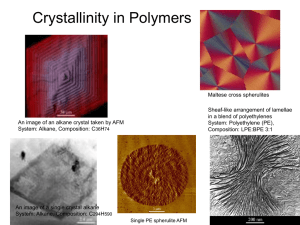
Crystalline polymers
• Crystallinty is areas in
polymer where chains
packed in a regular way.
• Both amorphous and
crystalline areas can exist
in the same polymer.
• More crystalline polymer
causes stronger and less
flexible polymer.
Melting temperature (Tm)
• The (Tm) when applied to polymers
suggests not a solid-liquid phase transition,
but a transition from a crystalline phase to a
solid amorphous phase. Crystalline melting
is only discussed with thermoplastics, as
thermosets will decompose at high
temperatures rather than melt.
Glass transition temperature (Tg)
The glass transition temperature (Tg)
describes the temperature at which
amorphous polymers undergo a second
order phase transition from a rubbery,
viscous amorphous solid (fresh spaghetti) to
a brittle, glassy amorphous solid (3 days old
Spaghetti)
Tensile strength
• The tensile strength of a material quantifies how much
stress the material will endure before failing. In general
tensile strength increases with polymer chain length.
Tensile strength
• Mechanical behavior of amorphous and semicrystalline polymers is strongly affected by Tg
• In general
• Polymers whose Tg is above the service
temperature are strong, stiff and sometimes brittle
• e.g. Polystyrene (cheap, clear plastic drink cups)
– Polymers whose Tg is below the service temperature
are weaker, less rigid, and more ductile
• Polyethylene (milk jugs)
Polymer additives
Before its conversion into plastic products,
polymer
resins
is
almost
always
compounded with various additives of
different nature, meant to improve
processing , stability, or mechanical
specifications.
Plasticizers
Are small molecules which occupy position
between polymer chains (like adding
water to mud to make it easy in molding)
1. To increase flexibility, elongation and to
reduce hardness and stiffness.
2. To lower the processing temperature
(energy saving, decomposition preventing)
Plasticizers
Plasticizer properties:
•
•
•
•
•
•
•
Low viscosity
High stable towards water and oils.
Low vapor pressure (Bpt is high).
Stable towards light and heat.
Low toxic.
Compatible.
Colorless.
Stabilizers
• Heat stabilizers (Pd soap, dibasic
phosphate).
• Antioxidants (easy to oxidize-phenols).
• UV absorbants.
• Light stabilizers: carbon black 2% = (1 to
20 years).
Fillers
Improves the attitude and lowers the cost (fiber , powder).
• Max. improvement for physical properties.
• Low water absorbance.
• Low specific gravity.
• High polymer wetting.
• Free from abrasives.
• Cheap and available.
• Odorless.
• Color compatible.
Polymer additives
• Blowing agents: physical, chemical.
• Antistatic agents: ethoxilated ammins.
• Lubricants: to allow easier processing and to
slides through dies easier {stearates}.
• Colorants: dyes or pigments.
• Reinforcing agents: (glass fiber, kevler).
• Flame retardants: (Cl/F and Br).
• Odorants
Forming of polymers
•
Polymeric materials are normally fabricated at
elevated temperatures and often by application
of high pressures.
• The technique used to form a particular polymer
depends on :
1. Whether it is thermoplastic or thermosetting.
2. The geometry and size of the final product.
Compression moldings
Both thermoplastic and thermosets can be formed by
compressing molding. Squeeze molten polymer between
hydraulic press.
Injection molding
In injection molding , polymer granules are
Compressed by a ram or a screw
Injected until molten.
Thermoplastic extrusion
Blow moldings
• Not to be confused with film blowing
( which is an extrusion-based process).
Thank you