c4 polymerization
advertisement

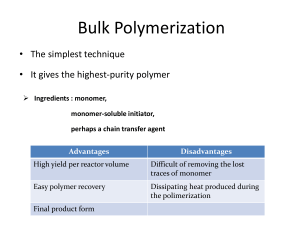
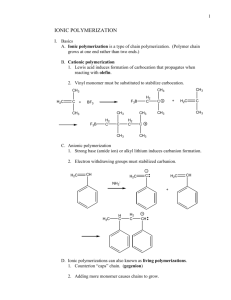
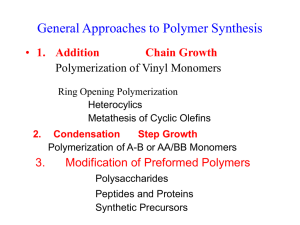
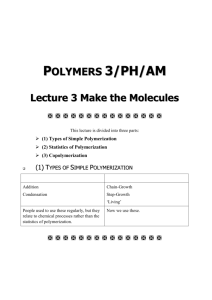
Polymerization reactions chapter 4. Fall 2011 1 outline • • • • • • • • Introduction Classifications Chain Polymerization (free radical initiation) Reaction Mechanism Kinetic Rate Expressions Definition of a Rate Equation Rate Expressions for Styrene Polymerization QSSA (Quasi-steady state assumption) chapter 4. Fall 2011 2 • we start an engineering discussion of polymers by addressing how they are made • beyond the selection of the monomer building blocks, the polymerization process is most important to properties: it sets the configuration • you should be able to model polymerizations and determine the effects of changing monomers, temperature, pressure and other variables chapter 4. Fall 2011 3 classifications Chain Polymerization (example: polystyrene) • monomer is added to the active center • high polymer is made in small quantities continuously • monomer concentration is decreased slowly • high molecular weight polymers are made when the concentration of active centers is low chapter 4. Fall 2011 4 • • • • Step Polymerization (example: nylon 6,6) ·end groups of the monomers react ·monomer is depleted rapidly ·high molecular weight polymer is made slowly • chapter 4. Fall 2011 5 Chain polymerization (free radical initiation) · · · · · · · monomers have double bonds typical monomers shown in Table 4.2 bulk: only monomer present emulsion: latex particles < 1 micron suspension: particles between 50 to 500 microns solution: monomer is dissolved in a second liquid particle morphology has commercial value chapter 4. Fall 2011 6 Reaction mechanism • We will learn a generic reaction mechanism which can be modified to describe many chain polymerization. Each step can be described by a reaction rate expression. The overall reaction rate model gives us the change in the monomer concentration with time, which can be used for process control. • • • . chapter 4. Fall 2011 7 Initiation (formation of free radicals) [initiators, catalysts] Benzoyl peroxide The radical can react with a double bond, linking the initiator fragment with the monomer. The reactive site moves to the end of the chain C O* + C O C* O O styrene chapter 4. Fall 2011 polymer active center 8 Propagation The active center adds monomer, transfer the radical to the new unit, and continues. chapter 4. Fall 2011 9 Termination C* + C* polymer chapter 4. Fall 2011 10 Reaction Kinetics • • • • • • • • • • • • • • • The minimum set of reactions which describe a free radical polymerization are: initiation, propagation, and termination. More complex systems could include: multiple initiation, propagation or termination steps, or could include side reactions such as: chain branching, monomer or polymer degradation, chain transfer, etc. chapter 4. Fall 2011 11 We will write general equations for simple systems, and you should be able to add as much complexity as you want for a specific system. chapter 4. Fall 2011 12 chapter 4. Fall 2011 13 chapter 4. Fall 2011 14 chapter 4. Fall 2011 15 We have generated four rate equations which describe a simple polymerization. The one which relates directly to monomer loss is the propagation reaction. We can solve this equation if we have an expression for M*, the free radical chain end concentration. We apply the quasi-steady state assumption in order to approximate M*. QSSA (Quasi-steady state assumption) If we want long chains, we need to have only a few of them reacting at one time. Therefore, we want M* to be small. We design most free radical polymerizations so that M* is much smaller than M. We make the approximation that the change in M* is nearly zero compared to the change in M. chapter 4. Fall 2011 16 chapter 4. Fall 2011 17 chapter 4. Fall 2011 18 chapter 4. Fall 2011 19 A photopolymerization case study POLY(METHYL VINYL ETHER) chapter 4. Fall 2011 20 chapter 4. Fall 2011 21 Objective: use a typical study of photoinitiator decomposition to estimate kd, the dissociation rate constant. Approach: use a system linked to vinyl ether polymerizations (solvents, monomers, etc. all affect the performance of catalysts, initiators and ionic catalysts) Reference: Cook, et al., Photopolymerization of vinyl ether networks using an iodonium initiator – the role of phototsensitizers, J. Polym. Sci., Part A: Polym. Chem., 47, 5474-87 (2009). Copy on course webpage. System: triethylene glycol divinyl ether; diphenyl iodonium salt, one of three photosensitizers (CPTXO, AO, CQ – not consumed). Note: photosensitizer allows the use of the visible spectrum range rather than UV (which would require quartz windows, etc). PHOTOINITIATOR DECAY chapter 4. Fall 2011 22 Absorbance change during irradiation specific wavelengths linked to fct. Groups (Fig. 4) 2 systems chapter 4. Fall 2011 23 PI rate constants photoinitiator Kd, s-1 AO 0.0374 CPTXO 0.0209 Polymerization conditions: 20 C; TEGDVE – triethylene glycol divinyl ether; 60 kJ/mol – heat of polymerization; chapter 4. Fall 2011 24 chapter 4. Fall 2011 25 Goofy stuff Degradation rate of CPTXO does not follow exponential decay over long times. As suggested on p. 5484, PI process is in competition with a side reaction that quenches CPTXO or with a process that consumes cations (perhaps an impurity). chapter 4. Fall 2011 26 Objective: use a typical study of MVE polymerization vs. T to to estimate Ea, the activation energy of the overall reaction process. This can be used to scale the polymerization rate vs. T for process design purposes Approach: use a system linked to vinyl ether polymerizations (solvents, monomers, etc. all affect the performance of catalysts, initiators and ionic catalysts) Reference: MVE in toluene; diethoxyethane/trimethyl silyl iodide, ZnI2 activator MVE POLYMERIZATION RATES VS. T chapter 4. Fall 2011 27 Semi-batch analysis Batch analysis of the rate can be done at the end of the monomer feed phase Each curve is modeled by an ionic polymerization eqn., yielding kp. These are plotted as kp vs. 1/T, and the slope is related to the activation energy. chapter 4. Fall 2011 28 chapter 4. Fall 2011 29 chapter 4. Fall 2011 30 Polymerization rates • Polymer Handbook: kp2/kt, • Chen et al.: 1.5 to 2 hours, 30 C, palladium complex • Sakaguchi et al.: 30 min, -78 C chapter 4. Fall 2011 31 chapter 4. Fall 2011 32