U1 Periodic Trends Homework Notes
advertisement

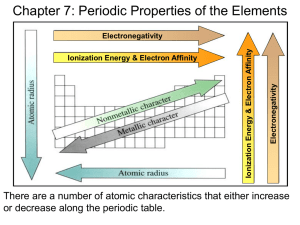
Chemistry Homework Read pp. 132-141 Problems p. 141 #1,2,3,5,10,12,13,14 1. What is ionization energy? A: (p. 133) Ionization energy is the energy required to remove an electron from an atom or ion. Note: Figure 16 on p. 133 shows the ionization of lithium. The picture below shows the ionization of sodium. Both reactions require some amount of ionization energy. 2. Why is measuring the size of an atom difficult? A: (p. 135) An atom’s size depends on the volume (amount of space) the electrons take up around the nucleus. The electron cloud has no clear edge. Also, the size of the electron cloud can change based on the chemical and physical environment. Notes: Figure 19 on p. 135 shows how atomic radius is measured. 3. What can you tell about an atom that has high electronegativity? A: (p. 137) Atoms with high electronegativity pull electrons towards them more than atoms with low electronegativity. Notes: Figure 22 and 23 show the trends for electronegativity. In the picture below, the Oxygen (O) is more electronegative (pulls harder on electrons) than Hydrogen (H). 5. What periodic trends exist for ionization energy? A: (p. 133-134) Ionization energy decreases as you move down a group. Ionization energy increases as you move across a period from left to right. Notes: Figure 17 and 18 show the ionization energy trends. Compare the chart below to Figure 17. Do they show the same trend? 10. What periodic trends exist for electronegativity? A: (p. 137-138) Electronegativity decreases as you move down a group. Electronegativity increases as you move across a period from left to right. Notes: Figure 22 and 23 show the ionization energy trends. Compare the chart below to Figure 22. Do they show the same trend? Compare to the ionization energy trend. 12. Explain why the noble gases have high ionization energies. A: (p. 134) The noble gases have high ionization energies because their outer electron shell (energy level) is full. This is a stable state and it takes a lot of energy to make an atom unstable. Also, in comparison to other atoms in the same period, noble gases have the maximum number of protons pulling on electrons that are a similar distance away from the nucleus. Notes: The second paragraph on p. 134 explains this idea. 13. What do you think happens to the size of an atom when the atom loses an electron? Explain. A: (p. 136, 139) The atom’s radius decreases. The size of the atom gets smaller. When an electron is taken away the atom becomes a positively charged ion. There are more protons than electrons. This uneven positive charge pulls the negatively charged electrons that are left closer to the nucleus. Also, if the electron that was taken was the only one in the outer energy level, there is bigger decrease in size because there are less energy levels. Notes: To figure this out, combine the discussion about decreasing atomic radius going across a period with the discussion about ionic radius. Figure 16 also helps! 14. With the exception of the noble gases, why is an element with a high ionization energy likely to have a high electron affinity? A: (p. 133, 134, 139) Atoms with high ionization energies are very attracted to their electrons. They want to keep them. It takes a lot of energy to pull them away. Similarly, atoms with high electron affinity are attracted to electrons. They also want electrons. This is not true for noble gases because noble gases just want to stay the way they are. Notes: To figure this out, combine the reasoning behind ionization energy and electron affinity.