Unit 1 – Atomic Theory and Structure
advertisement

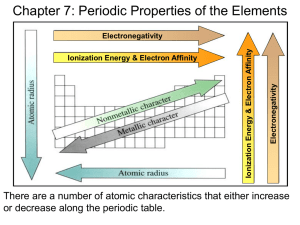
Unit 1 – Atomic Theory and Atomic Structure Section 4 – Periodic Trends The Periodic Table 1. List the diatomic elements. Hydrogen, Nitrogen, Oxygen, Fluorine, Bromine, Chlorine, Iodine (THERE ARE 7) 2. List the elements that are gases at room temperature. Hydrogen, Helium, Nigrogen, Oxygen, Fluorine, Neon, Chlorine, Argon, Krypton, Xenon, Radon 3. List the elements that are liquids at room temperature. Mercury and Bromine (Gallium comes close.) 4. List the metalloids. Boron, Silicon, Germanium, Arsenic, Antimony, Tellurium, Polonium (THERE ARE 7) Atomic Radius 5. Explain why the atomic radius of Li is larger than that of Be. Lithium has three protons and beryllium has four protons (both have the same number of energy levels containing electrons) so the effective nuclear charge (Zeff) is higher in beryllium than in lithium, which causes the electron cloud to be pulled closer to the nucleus, resulting in a smaller atomic radius. 6. Explain why atomic size decreases from Na to Cl in the periodic table. Na and Cl are both in the same periodic on the periodic table, and as one moves to the right in a row on the period table, the Zeff increases which pulls in the electron cloud and decreases the radius of the atom. 7. The Ca2+ and Cl- ions are isoelectronic, but their radii are not the same. Which ion has the larger radius? Explain. Chloride has a larger radius because the calcium ion has a larger effective nuclear charge (Z eff) due to the fact that it has more protons. The higher effective nuclear charge in the calcium is going to cause it to have a smaller radius because the electron cloud is pulled closer to the nucleus. 8. Consider the two chemical species S and S2-. a. Write the electron configuration of each species. S: 1s22s22p63s23p4 S2−: 1s22s22p63s23p6 Note: Replacement of 1s22s22p6 by [Ne] is acceptable. b. Explain why the radius of the S2- ion is larger than the radius of the S atom. The nuclear charge is the same for both species, but the eight valence electrons in the sulfide ion experience a greater amount of electron-electron repulsion than do the six valence electrons in the neutral sulfur atom. This extra repulsion in the sulfide ion increases the average distance between the valence electrons, so the electron cloud around the sulfide ion has the greater radius. c. Which of the two species would be attracted to a magnetic field? Why? The sulfur atom would be attracted into a magnetic field. Sulfur has two unpaired p electrons, which would interact with an external magnetic field, causing a net attraction into the field. The sulfide ion would not be attracted into a magnetic field because all the electrons in the species are paired, meaning that their individual magnetic moments would cancel each other. Ionization Energy 9. Explain why the ionization energy of nitrogen atoms is higher than expected. Nitrogen atoms have a half-filled set of p-orbitals. Half-filled sets of orbitals have an increased stability. 10. Explain why the second ionization energy of K is greater than the second ionization energy of Ca. It is easier to remove the second electron from calcium because after the second electron has been removed, the ion has a noble gas configuration. Removing one electron from K is relatively easy (low energy required), but removing the second is very difficult because it is at the noble gas configuration, and very stable after removing one electron. 11. The first ionization energy of Be is 900 kJ/mol, but the first ionization energy of B is 800 kJ/mol. Explain. The trend suggests that as one moves to the right in a row on the periodic table, the ionization energy increases (because the Z eff or the pull of the nucleus increases). This is slightly different for Be and B because Be has a full s shell and is not going to easily break up the two electrons in it. Boron’s outer electron is alone in the 2p shell and is therefore easier to remove (easier meaning it requires less energy). The s electrons are held more tightly to the nucleus than the p electrons. 12. Explain why the first ionization energy of K is less than that of Na. Potassium (K) has four energy levels of electrons and sodium (Na) only has three. As the number of energy levels increases, the distance in which the nucleus must pull the electrons increases. The farther away the electrons are from the nucleus, the less energy is required to remove the electron. 13. Using principles of atomic and molecular structure and the information in the table below, answer the following questions about atomic fluorine, oxygen, and xenon, as well as some of their compounds. Atom First Ionization Energy (kJ/mol) F 1,681.0 O 1,313.9 Xe ? a. Write the equation for the ionization of atomic fluorine that requires 1,681.0 kJ/mol. F(g) F+(g) + e- b. Account for the fact that the first ionization energy of atomic fluorine is greater than that of atomic oxygen. In both cases the electron removed is from the same energy level (2p), but fluorine has a greater effective nuclear charge due to one more proton in its nucleus (the electrons are held more tightly and thus take more energy to remove). c. Predict whether the first ionization energy of atomic xenon is greater than, less than, or equal to the first ionization energy of atomic fluorine. Justify your prediction. The first ionization energy of Xe should be less than the first ionization energy of F. To ionize the F atom, an electron is removed from a 2p orbital. To ionize the Xe atom, an electron must be removed from a 5p orbital. The 5p is a higher energy level and is farther from the nucleus than 2p, hence it takes less energy to remove an electron from Xe. 14. The S- ion is isoelectronic with the Ar atom. From which species, S 2-or Ar, is it easier to remove an electron? Explain. It requires less energy to remove an electron from a sulfide ion than from an argon atom. A valence electron in the sulfide ion is less attracted to the nucleus (charge +16) than is a valence electron in the argon atom (charge +18). Metals, Nonmetals, and Metalloids 15. Carbon and lead are in the same group of elements but carbon is classified as a nonmetal and lead is classified as a metal. Explain. Carbon and lead are in the same group of elements but carbon is classified as a nonmetal and lead is classified as a metal. 16. List some characteristics of metals, nonmetals, and metalloids. Most elements are metals. They are usually shiny, very dense, and only melt at high temperatures. Their shape can be easily changed into thin wires or sheets without breaking (malleable and ductile). Metals will corrode. Heat and electricity travel easily through metals. Nonmetals, on the right side of the periodic table, are very different from metals. Their surface is dull and they don’t conduct heat and electricity. As compared to metals, they have low density and will melt at low temperatures. The shape of nonmetals cannot be changed easily because they are brittle and will break. Elements that have properties of both metals and nonmetals are called metalloids. They can be shiny or dull and their shape is easily changed. Electricity and heat can travel through metalloids but not as easily as they travel through metals. Other Trends and Review 17. Which group or groups (1A, 2A, 6A, 7A, and 8A) have the following descriptions? a. __1A__ Easily loose one electron b. __8A __ All gases at room temperature c. __7A __ Easily gain one electron d. __6A __ Tendency to gain two electrons e. __2A __ Alkaline earth metals f. __1A __ React vigorously with water to form a base g. __6A __ Chalcogens h. __1A __ React vigorously with water to produce hydrogen i. __1A 2A __ All solids at room temperature j. __2A __ First and second ionization energies are low k. __1A __ Are the largest elements in their period l. __1A __ Alkali metals m. __8A __ Most unreactive n. __1A __ Only first ionization energy is very low 18. Compounds containing Kr have been synthesized, but there are no known compounds that contain He. Explain. Helium has a full shell (n=1 is full) so it wont react. Kr has an unfilled 4p and 4d subshells (even though the 4s and 4p subshells are full) so Krypton is more likely to react.