Periodic Trends
advertisement

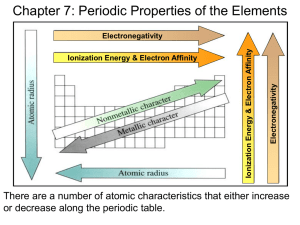
Special Topics for SOL 2 rd 3 Power Point Periodic Trends (Chap 14) Shorthand Electron Configurations Shorthand configurations are a useful tool. Let’s look at an example for Y, Z=39 The electron configuration for yttrium is 1s22s22p63s23p64s23d104p65s24d1 To do a shorthand configuration, we use the noble gas preceding the element and we put that in brackets (the bold and italics part) That’s Kr and then we also just write whatever is left over. [Kr] 5s24d1 You Try… Do a shorthand configuration for Fe Br Rb The Answers… Do a shorthand configuration for Fe = [Ar]4s23d6 Br = [Ar]4s23d104p5 Rb = [Kr]6s1 Objective B http://www.rsc.org/chemsoc/visualelements/PAGES/data/intro_groupvii_data.html Notice that the halogens all have an ending configuration of ns2np5. That means they have 7 valence electrons. F Br At [He]2s22p5 Cl [Ar]3d104s2 4p5 I [Xe]4f14 5d106s2 6p5 [Ne]3s23p5 [Kr]4d105s2 5p5 Similarly, alkali metal have 1 valence electron. Noble gases have 8, etc. All transition metals have 2. Objective B All of the transition metals have 2 valence electrons, with 2 exceptions. “d” electrons are not valence electrons. Why not? Transition metals are where the d orbitals are being filled up. Here are the electron configurations for all of them. Sc V Mn Co Cu [Ar]3d14s2 [Ar]3d34s2 [Ar]3d54s2 [Ar]3d74s2 [Ar]3d104s1 Ti Cr Fe Ni Zn [Ar]3d24s2 [Ar]3d54s1 [Ar]3d64s2 [Ar]3d84s2 [Ar]3d104s2 Objective B Notice that Cr and Cu are “exceptions.” They both have 1 valence electron. They do this because in the case of Cr, moving an electron from the 4s level to the 3d level gives us a half full set of d orbitals. That’s more stable than if Cr would have followed the pattern, and ended with “4s23d4” Cr [Ar]3d54s1 Objective B Similarly, Cu has 1 electron in the 4s energy level and 10 in the 3d level, because having a full set of d electrons is also more stable. Cu [Ar]3d104s1 Objective B The “inner transition metals” are the lanthanide and actinide series. That’s where the f electrons are filled up. That’s about all I’m going to say about that. Objective C The periodic table allows you to predict trends in certain properties. Get out a periodic table and put these trends as notes on your periodic table. The first trend is Atomic radius. Atomic radius is the size of the atom. It’s defined as ½ the distance between two nuclei which are bonded together. Objective C Ionic radius is another property It is the size of an ion. Ionic radius is fairly similar to atomic radius. A positive ion is also called a CATION. A negative ion is also called an ANION. A cation is always smaller than the from. atom it is formed An anion is always larger than the atom it is formed from. Objective C http://www.chem1.com/acad/webtext/atoms/atpt-images/ionic_radii.jpg Since cations lose electrons to form positive ions and anions gain electrons to form negative ions, it should make sense that they are SMALLER than the atom. Objective C Ionization energy is the amount of energy required to remove an electron from a gaseous atom. The energy required to remove the first electron is called the FIRST IONIZATION ENERGY. The energy required to remove the second electron is the second ionization energy. And so on… Metals always have LOWER ionization energies than nonmetals. That is because metals tend to lose electrons and nonmetals tend to gain them. Objective C It is VERY MUCH easier to remove a valence electron (an electron in the highest energy level) than an “inner core” electron. The inner core electrons are ANY electrons which are not VALENCE electrons. Na = 1s22s22p63s1 White = inner core electrons and Blue = Valence electrons Objective C http://www.knowledgerush.com/wiki_image/8/87/LinusPauling.jpeg Electronegativity is measured on a scale from 0.0 to 4.0. By definition, F is the most electronegative element at 4.0. Nonmetals have a high electronegativity. Metals have a low electronegativity. Electronegativity Think of this as the “greediness” of an atom not only holding on to it’s own electrons, but ALSO wanting to “steal” electrons from other atoms. The Trends Atomic Radius AND Ionic Radius increase as you go down a group. Atomic Radius AND Ionic Radius decrease as you go from left to right across a period. Electronegativity AND Ionization Energy decrease as you go down a group. Electronegativity AND Ionization Energy increase as you go from left to right across a period. Note the trends are opposites. Draw some arrows on your periodic table to help you remember the trends. The End