Electrochemistry Lab Report: Redox Potentials & Cell Potential
advertisement
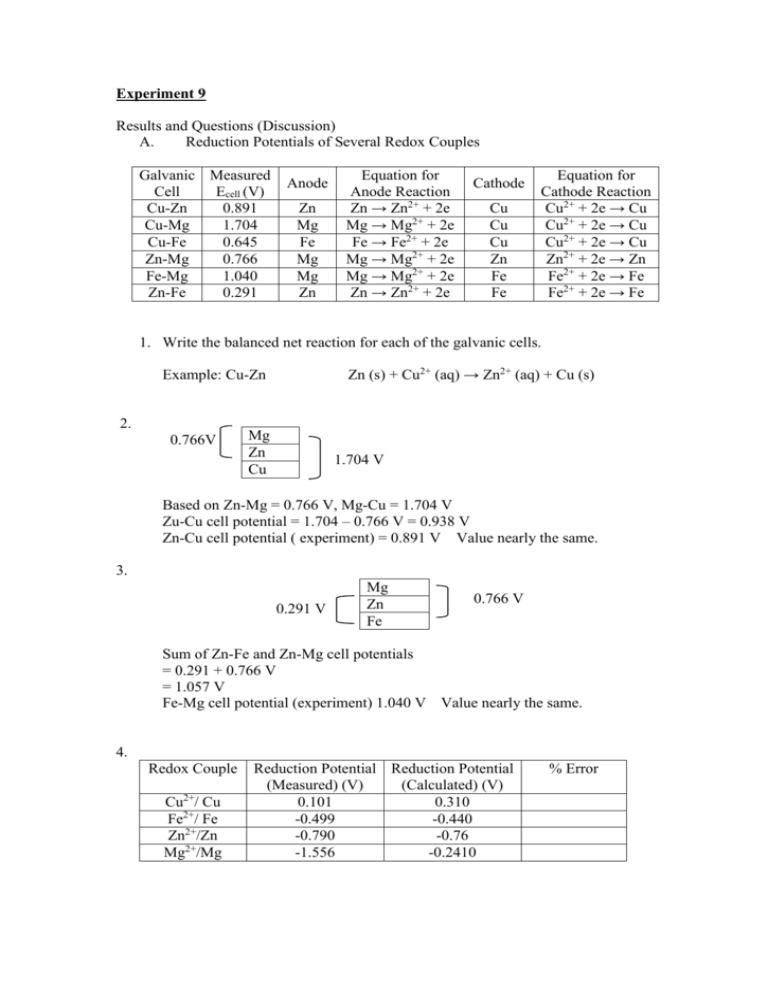
Experiment 9 Results and Questions (Discussion) A. Reduction Potentials of Several Redox Couples Galvanic Measured Cell Ecell (V) Cu-Zn 0.891 Cu-Mg 1.704 Cu-Fe 0.645 Zn-Mg 0.766 Fe-Mg 1.040 Zn-Fe 0.291 Anode Zn Mg Fe Mg Mg Zn Equation for Anode Reaction Zn → Zn2+ + 2e Mg → Mg2+ + 2e Fe → Fe2+ + 2e Mg → Mg2+ + 2e Mg → Mg2+ + 2e Zn → Zn2+ + 2e Cathode Cu Cu Cu Zn Fe Fe Equation for Cathode Reaction Cu2+ + 2e → Cu Cu2+ + 2e → Cu Cu2+ + 2e → Cu Zn2+ + 2e → Zn Fe2+ + 2e → Fe Fe2+ + 2e → Fe 1. Write the balanced net reaction for each of the galvanic cells. Zn (s) + Cu2+ (aq) → Zn2+ (aq) + Cu (s) Example: Cu-Zn 2. 0.766V Mg Zn Cu 1.704 V Based on Zn-Mg = 0.766 V, Mg-Cu = 1.704 V Zu-Cu cell potential = 1.704 – 0.766 V = 0.938 V Zn-Cu cell potential ( experiment) = 0.891 V Value nearly the same. 3. 0.291 V Mg Zn Fe 0.766 V Sum of Zn-Fe and Zn-Mg cell potentials = 0.291 + 0.766 V = 1.057 V Fe-Mg cell potential (experiment) 1.040 V Value nearly the same. 4. Redox Couple Cu2+/ Cu Fe2+/ Fe Zn2+/Zn Mg2+/Mg Reduction Potential Reduction Potential (Measured) (V) (Calculated) (V) 0.101 0.310 -0.499 -0.440 -0.790 -0.76 -1.556 -0.2410 % Error Sample Calculation for Reduction Potential (Measured) (V) (a) Ecell = Ecathode - Eanode ECu2+/Cu – EZn2+/Zn = Ecell ECu2+/Cu – (-0.79) = 0.891 V ECu2+/Cu = 0.107 V Sample Calculation for Reduction Potential (Calculated) (V) 0.0592 [ Zn 2 ] (a) Ecell = Eocell log 2 [Cu 2 ] ECu2+/Cu – EZn2+/Zn = EoCu2+/Cu – EoZn2+/Zn - ECu2+/Cu – EZn2+/Zn = [0.34- (-0.76)] - 0.0592 [ Zn 2 ] log 2 [Cu 2 ] 0.0592 [0.1 ] log 2 [0.1 ] ECu2+/Cu – (-0.79) = 1.1 ECu2+/Cu = 0.31 V These 2 values have to find from Standard Reduction Potential Table from you text book. % Error Cu2+/Cu = B. 0.31 0.101 100% 0.31 = 67.21 % Effect of Concentration Changes On Cell Potential 1. Cell Potential of “Concentrated Cell” = 0.051 V Anode reaction : 1 M CuSO4 Cathode reaction : 0.001 M CuSO4 A potential is recorded because of the difference in the solutions concentration. 2. Cell Potential from complex formation: 0.445 V Anode: Cathode: Potential changed with the addition of NH3 (aq) because there are more Cu2+ ions flow from cathode and the concentration of Cu2+ ions decrease. 3. Cell Potential from precipitate formation: 0.5 V Observation of solution in half cell: Potential changed with the addition of Na2S because of the formation of CuS released electrons. As there are more electrons flowing from anode to cathode, thus potential increased. 4. The cell potential would have decreased if NH3(aq) and/ or the Na2S(aq) had been added to 1 M CuSO4 instead of 0.001 M CuSO4 solution of the cell because the mole concentration of 1M CuSO4 has more copper than 0.001 M CuSO4 C. The Nerst Equation and An Unknown Concentration Solution No. 1 2 3 4 [Cu(NO3)2] (M) 0.1 0.001 0.00001 0.0000001 Log [Cu2+] -1 -3 -5 -7 Ecell (Measured) 0.962 V 0.874V Sample Calculation Cu(NO3)2 0.1 M (b) Ecell = Eocell - 0.0592 [ Zn 2 ] log 2 [Cu 2 ] ECu2+/Cu – EZn2+/Zn = EoCu2+/Cu – EoZn2+/Zn = 0.34 – (-0.76) = 1.1 V. 0.0592 [0.1 ] log 2 [0.1] Ecell (Calculated) 1.100 V Experiment 10 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 Trial 1 0.388 0.412 900 0.15 0.345 0.456 0.044 6.925 × 10-4 1.385 × 10-3 135 8.4375 × 1020 6.092 × 1023 Trial 2 0.375 0.456 900 0.14 0.328 0.503 0.047 7.397 × 10-4 1.479 × 10-3 126 7.875 × 1020 5.323 × 1023 5.708 × 1023 6.022 × 1023 - 5.22 % 9.748 × 104 8.517 × 104 9.132 × 104 9.65 × 104 - 5.36 % All Calculations follow the formulas in your lab manual.