7. Ionic Bonding
advertisement

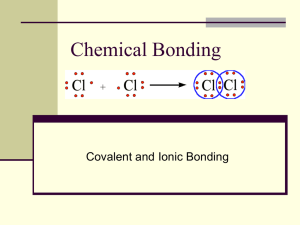
The Nonmetal-Nonmetal vs Metal – Nonmetal Bond Lewis Dot Structures • A famous chemist named Lewis invented a symbol to show valence electrons. He used a dot next to the symbol to represent each valence electron. The dots are spread around the 4 sides. • Each pair of dots Represents a bond. Covalent Bonds •What is a Covalent Bond? •? - A covalent bond is a chemical bond resulting from SHARING of electrons between 2 nonmetals. Covalent Bonds can have multiple bonds, so you should be familiar with the following… Single Covalent Bondchemical bond resulting from sharing of an electron pair between two atoms. H2O Double Covalent Bondchemical bond resulting from sharing of two electron pairs between two atoms. CO2 Triple Covalent Bondchemical bond resulting from sharing of three electron pairs between two atoms. N2 Types of Covalent Bonds • Two types of colvalent bonds: nonpolar and polar • Recall electronegativity (desire for electrons) -see shaded table on ole yeller • The electronegativity difference between the two atoms determines whether it is a nonpolar or polar bond. Electronegativity difference: 0 .4 Nonpolar Polar 2.0 Ionic Polar Bonds A nonpolar bond tends to share electrons equally A polar bond means there is a dipole or one pole (end) with a positive charge and one pole (end) with a negative charge, therefore they tend to stick together better since their opposite charges attract. (=)(-) Very strong polar bonds are ionic bonds like NaCl Covalent Bonds Do NOT have ions or need to Balance Charges • They use prefixes to show the number of atoms: • Mono• Di• Tri• Tetra- • Examples: • H2O = dihydrogen monoxide • CO2 = carbon dioxide • dinitrogen tetraoxide = N2O4 • Phosphorus trichloride = PCl3 Pop Quiz: Covalent Bonds: HOT or NOT? HOT, for sure! If Miley says it’s hot, it’s HOT! When a metal and nonmetal come together, a pair of electrons acts as a bond and they each become ions. Ionic Bonding Rules: • Metal first, nonmetal second • Nonmetal ion becomes ‘ide’ • Metal is positive, nonmetal is negative • Charges must balance to zero • Formula uses a subscript to balance charges • Example: MgCl2 ; Na2O; NaCl The sodium atom and chloride atom bond together as ions and form a new compound. This is called an ionic bond. Practice: Sodium and fluorine Barium and iodine lithium and phosphorus Aluminum and oxygen Beryllium and oxygen Calcium and nitrogen Answers: Sodium and Barium and fluorine iodine sodium fluoride barium iodide NaF BaI2 lithium and phosphorus lithium phosphide Li3P Aluminum and Beryllium and Calcium and oxygen sulfur nitrogen aluminum beryllium calcium nitride oxide sulfide Ca3N2 Al2O3 BeS Solutions • When ionic compounds are put in water, they dissolve into ions: Polyatomic ions: are groups of atoms bonded together with a charge hence the name “poly” “atomic” “ions”. • • • • • Examples: OH-1 = hydroxide NO3-1 = nitrate PO4-3 = phosphate SO4-2 = sulfate Practice: Use polyatomic ions just like any other ion; But when you have more than one , use parentheses. • barium hydroxide= (Notice parentheses show multiple ions.) barium hydroxide= Ba OH +2 -1 Ba(OH)2 +2 -1(2) = 0 • strontium nitrate = • strontium nitrate = Sr NO3 +2 -1 strontium nitrate = Sr(NO3)2 +2 + -1(2) • lithium phosphate • lithium phosphate = Li PO4 +1 -3 lithium phosphate Li3PO4 +1(3) + -3 = 0 potassium sulfate • potassium sulfate = K SO4 +1 -2 potassium sulfate = K2SO4 +1(2) + -2 = 0 Transition metals: Metals that have more than one possible charge: • • • • Cobalt: Co+2, Co+3 Copper: Cu+, Cu+2 Iron: Fe+2, Fe+3 Lead: Pb+2, Pb+4 • When writing the names, always use roman numerals to show the charge. Examples: • • • • Cobalt (II) Co+2, Cobalt (III) Co+3 Copper(I), Cu+, or Copper (II), Cu+2 Iron(II) Fe+2, or iron (III), Fe+3 Lead(II), Pb+2, or lead (IV), Pb+4 • Each different charged ion behaves completely different than the other! The charges matter! Practice! Lead (IV) hydroxide • Lead(IV) hydroxide = Pb(OH)4 • Copper (II) nitrate • Copper(II) nitrate = Cu(NO3)2 +2 -1 • CoPO4 • CoPO4 = Cobalt (III) phosphate +3 -3 • Fe2(SO4)3 +3 -2 Iron (III) sulfate Fe2(SO4)3 Hydrates Some ionic compounds absorb water molecules into their structures. These are called hydrates. NaCl•2H2O is the symbol for sodium chloride dihydrate. Notice the prefix di- means 2 water molecules. Name these hydrates: MgSO4•5H2O CuCl2•4H2O Finding Percentage of Water in a Hydrate You can find the percentage of water in a hydrate by dividing the mass of the water by the total mass times 100. Lets take NaCl•2H2O Using the periodic table, the mass of Na=23, Cl=35.5, H2O = 18. Total mass with 2 H20 is 94.4 Water mass (36)/ Total (94.4) x 100 = 38 % water What are the differences between ionic bonding and covalent bonding? List them below: Differences: • • • • Ionic Metal , nonmetal-ide Balance charges using subscripts Polyatomic ions use parentheses in multiples Transition metals use roman numerals to show charge Differences: • • • • • Covalent Two nonmetals Nonmetal, nonmetalide No ions- they share eUse prefixes Don’t need to balance Differences: • • • • Ionic Metal , nonmetal-ide Balance charges using subscripts Polyatomic ions use parentheses in multiples Transition metals use roman numerals to show charge • • • • • Covalent Two nonmetals Nonmetal, nonmetalide No ions- they share eUse prefixes Don’t need to balance Ionic bonds: HOT or NOT? Definitely HOT Baby!