39-49
advertisement
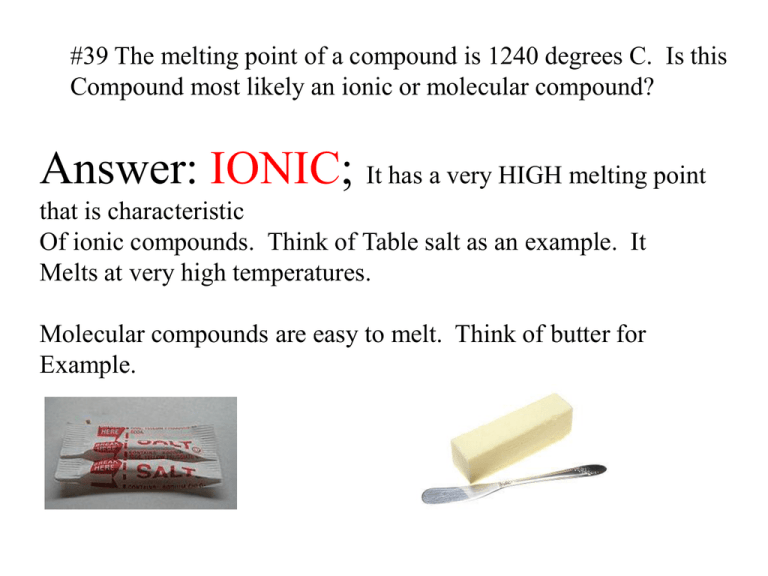
#39 The melting point of a compound is 1240 degrees C. Is this Compound most likely an ionic or molecular compound? Answer: IONIC; It has a very HIGH melting point that is characteristic Of ionic compounds. Think of Table salt as an example. It Melts at very high temperatures. Molecular compounds are easy to melt. Think of butter for Example. #40 Identify the number and kinds of atoms present in a Molecule of each compound: a. C6H8O6 Has 6 carbons, 8 hydrogen's, and 6 oxygen's. B. C12H22O11 Has twelve carbons, twenty two hydrogens, and Eleven oxygens C. C7H5N3O6 has seven carbons, five hydrogens, three nitrogens And six oxygens 41. Which of the following gases in Earth’s atmosphere Would you expect to find as molecules and which as Individual atoms? Explain. a. Nitrogen Nitrogen would be found as N2 with a triple Covalent bond. b. Oxygen Oxygen would be founds a O2 with a double Covalent bond. c. Argon Argon never bonds with anything including itself So you would expect it to be individual atoms. #42 Explain why neon is monatomic but chlorine is diatomic. Neon is monatomic (means one atom) because it is a noble gas And doesn’t bond with anything. Chlorine is diatomic (meaning two atoms) Cl2 because Each chlorine needs one electron to fill its outer valence shell It does so by sharing one electron with another chlorine. #43 Classify the following compounds as ionic or covalent Non-polar (totally equal sharing) Ionic (total Transfer) #43 Classify the following compounds as ionic or covalent Non-polar (totally equal sharing) Ionic (total Transfer) #44 Describe the difference between an ionic and a covalent bond? #45 How many electrons do two atoms in a double bond Covalent bond share? How many in a triple covalent bond? Double bond Shares four triple bond Shares six #46 Write plausible electron dot structures for the following Substances. Each substance contains only single covalent bonds. I2 H2S OF2 NI3 #47 Characterize a coordinate covalent bond and Give an example: A coordinate covalent bond occurs when one atom Supplies both of the electrons in the bond. In this example Nitrogen supplies both electrons to the hydrogen that comes in to bond. In this example fluorine Brings in both electrons To bond with Boron. #48 Explain why compounds containing C – N and C – O single bonds can form coordinate covalent bonds With H+ but compounds containing only C – H and C – C single bonds cannot. In the case of C – N bonds and C – O bonds, an unshared pair Of electrons can exist to allow Another atom to bond without Bringing in two of its own. C – H and C – C bonds don’t Usually have unshared pairs to Bond with. #49 Using electron dot structures, draw at least two Resonance structures for the nitrite ion (NO21-)