Denaturation of Proteins
advertisement

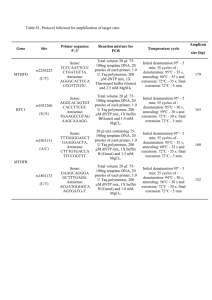
Denaturation of Proteins What is Denaturation? When the unique 3-D structure of proteins is destroyed. It leads to temporary or permanent loss of activity. When and How are Proteins Denatured? At Very High or Low pH. At Very High Temperatures. By Heavy Metal Ions. By Small Polar Molecules. Denaturation at HIGH or LOW pH Affects Ionic Bonds. A High (>9) or Low pH (<3) will neutralise the charge on one of the ionically bonded ions. Hence the ionic bond is broken. Denaturation at High Temperatures As the temperature increases the energy of the protein increases. As the energy increases bonds are broken in the following order: • Van der Waal’s • Hydrogen Bonds • Ionic Bonds Denaturation by Metal Ions Certain metal ions will disrupt the Van der Waals’ forces in proteins. Metal Ions, such as Ag+ and Hg2+, also inhibit enzyme activity. Heavy They do so by reducing the SH groups in cysteine. PLEASE NOTE: Spelling mistake on pictures: --- The S-S bond is a DISULPHIDE bond. Denaturation by Small Polar Molecules Urea ( CO(NH2)2 ) in concentrated solution will denature proteins. It disrupts the Hydrogen Bonds. This causes complete denaturation. Everyday Examples of Denaturation When milk curdles, the acidity increases. Thermal denaturation by cooking. Mechanical denaturation when whisking an egg. Perming hair breaks then reforms the disulphide bonds. THE END Thank you for listening. This presentation, along with the one on Haemoglobin, will be available on my website: www.alexridge.tk