An Evaluation of the Thermodynamics of
advertisement

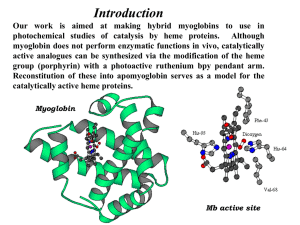
An Evaluation of the Thermodynamics of Unfolding of Myoglobin Cortney Paige Thomasson Longwood University, Department of Chemistry and Physics, 201 High Street, Farmville, VA 23909 Abstract: The purpose of this experiment was to determine the thermodynamic parameters associated with the unfolding, or denaturation, of myoglobin using a strong denaturant, guanidine-hydrochloric acid (GuHCl), and absorption spectroscopy. The heme group of myoglobin absorbs light at 409 nm when in its native, folded state. Absorbance was recorded of the myoglobin solution at three different temperatures as the concentration of GuHCl was increased, causing myoglobin to unfold. From the initial absorbance data, the transition region where both the native and unfolded forms of myoglobin exist at the same time was analyzed to determine the free energy change in water, the rate of change of the free energy of denaturation with respect to the concentration of denaturant, and the equilibrium constant for this reaction. The free energy change values found through experimentation ranged between 30-53.8 kJ/mol. Experimental values were then compared to previous literature values. Introduction Results/Discussion • • Myoglobin should unfold with a lower concentration of GuHCl as temperature increases. • Transition region is where both native and unfolded states of the protein exist. • Equilibrium constant is calculated using absorbance values of the transition region, folded state, and unfolded state. • Figures below show the values for the rate of free energy of denaturation (slope) and the free energy of this process in pure water (intercept) Protein Folding and Unfolding − Folded state = native conformation (functional 3D shape) − Unfolded state = denaturation (primary structure of linear chain of amino acids) − Guanidine-hydrochloric acid (GuHCl) is a strong protein denaturant • Myoglobin1 − Monomeric globular protein in muscle tissue − Comprised of 154 amino acids − 8 alpha helices connected by loops − Function is to bind to oxygen − Heme Group = iron atom in center surrounded by nitrogen atoms − Heme group absorbs light at 409 nm when protein is folded − Does not absorb light when denatured Experimental • • • • • • Three stock solutions were prepared: 0.05 M potassiumphosphate buffer with pH=7.01, 1 mg/mL freeze dried equine heart myoglobin in buffer, and 8 M GuHCl. Spectra were collected for a series of prepared solutions with constant myoglobin concentration of 0.20 mg/mL and varied GuHCl concentration from 0-3 M. Solutions were prepared by adding the appropriate amount of GuHCl to a 10 mL volumetric flask, followed by buffer, then myoglobin. Myoglobin was always added after the GuHCl/buffer solution due to the fact that myoglobin would completely denature is GuHCl was not diluted first. Three sets of solutions were made and incubated for 30 minutes at 15oC, 22oC, and 32oC. After incubation, the UV-vis spectra of each solution were collected using a Vernier SpectroVis Plus Spectrophotometer set to monitor absorbance versus concentration of GuHCl at a wavelength of 409 nm. Figure 4: Rate of change of free energy of denaturation at 15o C. Figure 1: Structure of Myoglobin2 Figure 2: Structure of Heme Group3 Results/Discussion Figure 5: Rate of change of free energy of denaturation at 22o C. Figure 3: Absorbance versus [GuHCl] plot Temperature ∆𝐇 𝐎 𝐆𝐨 Figure 6: Rate of change of free energy of denaturation at 32o C. m (J/mol) Cm (M) K 𝟐 (J/mol) 15oC 33800 27100 1.248 1.54 22oC 30000 23900 1.254 1.44 32oC 53800 38400 1.402 14.7 Table 1: Thermodynamic parameters calculated from results References 1. Myoglobin. Wikipedia. http://en.wikipedia.org/wiki/Myoglobin (accessed March 7, 2014). 2. Watson, H.C. The stereochemistry of the protein myoglobin. RCSB protein data bank. http://www.rcsb.org/pdb/explore/explore.do?structureId=1MBN (accessed March 7, 2014). 3. Protoporphyrin IX Containing Fe. RCSB protein data bank. http://www.rcsb.org/pdb/ligand/ligandsummary.do?hetId=HEM&sid=1MBN (accessed March 7, 2014). 4. Sykes et al. J. Chem. Ed. 1999, 76, pp. 1283-1284. • Range of published values for ∆𝐇 𝐎 𝐆𝐨 range from 39-50 kJ/mol.4 𝟐 • Endothermic reaction because equilibrium constant increases with temperature. • Entropically driven reaction is favorable.