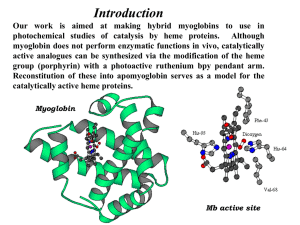
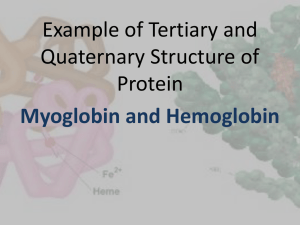
Theory of electron-vibrational and electron
advertisement

Theory of electron-vibrational and electron-conformational interactions in chemistry, biochemistry, biological and molecular physics; protein structure and dynamics; quantum chemistry; theoretical spectroscopy (optical absorption, resonance Raman scattering, nuclear magnetic resonance, Mössbauer effect); catalytic activity of transition metal complexes and metalloenzymes, especially of metalloporphyrins and heme proteins. Main Results Theoretical approach to understanding and prediction of structural and dynamic features of metalloporphyrins and heme proteins based on the Jahn-Teller and pseudo Jahn-Teller effects was developed. In particular 1. Influence of axial ligand coordination to 3dn metalloporphyrins on the metal position in respect to the porphyrin plane. The developed theory explained all the known experimental data and allowed to predict configuration of new complexes. Application of the developed theory to the heme structure of different heme proteins explained change in the iron atom position caused by coordination of different axial ligands and weak dependence of the heme structure of cytochrome c on the iron oxidation state. The developed theory also predicted specific change in the heme geometry upon Soret excitation, this result was proved experimentally lately. 2. Influence of the iron displacement out of the porphyrin plane on the resonance Raman and optical absorption spectra of deoxyheme proteins. It was obtained that position of the resonance Raman iron-histidine band and intensity of the optical absorption band III are controlled by the heme doming. This result allowed to explain the experimentally obtained temperature and pressure dependences of the shapes of both the bands. Also it was shown that temporal dependence of the resonance Raman iron-histidine band and optical absorption band III after the photolysis of the heme protein complexes allows to study separately heme and protein globule relaxations. 3. The differences in the coordination geometries of carbon monoxide, nitric oxide and dioxygen to different 3dn metalloporphyrins was shown to be a manifestation of pseudo Jahn-Teller effect. The same theory explained difference between yields of photolysis of these compounds and predicted essential population of high rotational states of photodissoated ligands, this prediction was supported experimentally lately. 4. The specific relationship between the splitting of the visible and Soret optical absorption bands of different porphyrins in a low-symmetry environment was shown to be controlled by the Jahn-Teller and pseudo JahnTeller interactions with low symmetry porphyrin vibrations. A method for investigation of activation of different molecules by metallocomplexes based on the vibronic theory of chemical activation and quantum chemical calculations of electron density metal→ligand transfer, was developed. This method was used to study activation of different diatomic ligands by iron porphyrin complexes, modeling active centers of different heme proteins. In particular 1. The stretching vibration of carbon monoxide, dioxygen and cyanide coordinated by different heme complexes in dependence on the coordination geometry and heme electrostatic environment. It was obtained that this frequency is strongly affected by the electric field, this result explained variation of the carbon monoxide band position in different heme proteins and their mutants. It was concluded that the shape of the carbon monoxide infrared absorption band must be sensitive to the dynamics of the heme environment, this conclusion was supported experimentally lately. 2. The experimental results on the temperature dependence of the carbon monoxide infrared absorption band of the corresponding complexes of different heme proteins in different solvents were interpreted using the theory of molecular absorption and taking into account the effect of the dynamics of the of amino acids forming the heme pocket and water molecules located in this pocket. It was shown that this dynamics is sensitive to the state of the protein environment, glassy matrix or solvent. 3. Electronic structures of thiolate and imidazolate iron porphyrin complexes (models of active centers of cytochrome P450 and horseradish peroxidase, respectively) with carbon monoxide and dioxygen were studied by using heme quantum chemical calculations. It was shown that deprotonation of the proximal iron ligand essentially affects the catalytic activities of these heme proteins and their optical absorption spectra. 4. Development of a theoretical model in frameworks of which experimental data on optical absorption, resonance Raman and Mössbauer spectroscopies, and magnetic susceptibility will be explained. Such a model is necessary to provide reliable interpretation of numerous spectroscopic data on the heme protein structure and dynamics.