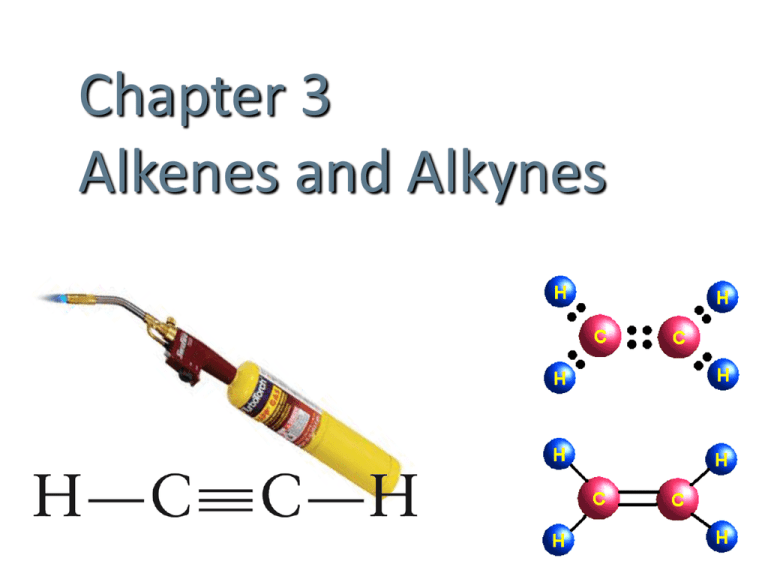
Chapter 3
Alkenes and Alkynes
Unsaturated Hydrocarbons
• Contain carbon-carbon multiple bonds.
Alkenes C=C double bonds
Alkynes C≡C triple bonds
Aromatics
benzene rings
12 Alkenes
• Structure:
• The VSEPR model predicts bond angles of 120° about
each carbon of a double bond.
H
121.7°
H
C C
H
H
Ethy lene
© 2006 Thomson Learning, Inc.
All rights reserved
12
H3 C
C C
H
Prop en
12-3
12 Alkenes
• Cis-trans isomerism
• because of restricted rotation about a carbon-carbon
double bond, an alkene with two different groups on
each carbon of the double bond shows cis-trans
isomerism.
CIS
© 2006 Thomson Learning, Inc.
All rights reserved
TRANS
12-4
12
(d)
(c)
© 2006 Thomson Learning, Inc.
All rights reserved
12-5
Problem 43, p. 355
12
Naming Alkenes
• Step 1: Name the longest chain that contains the C=C
bond. Use the IUPAC root and the –ene ending.
• Step 2: Number the longest chain so the C=C bond
gets the lowest number possible.
• Step 3: Designate the C=C bond in the name with the
lowest-numbered carbon.
© 2006 Thomson Learning, Inc.
All rights reserved
12-6
12
Examples:
1
2 3 4
CH3-CH=CH-CH3
2-butene
6
5 4
3
2 1
CH3-CH2-CH2-CH=CH-CH3
2-hexene
© 2006 Thomson Learning, Inc.
All rights reserved
12-7
12
Naming Alkenes, cont.
• Step 4: Locate and name attached groups.
• Step 5: Combine all the names as you did with
alkanes.
© 2006 Thomson Learning, Inc.
All rights reserved
12-8
12 Alkynes - IUPAC Names
• follow the same rules as for alkenes, but use the
ending -yne to show the presence of the triple bond.
1
CH3 CHC CH
CH3
2
4
3
3-Methyl-1-butyne
© 2006 Thomson Learning, Inc.
All rights reserved
CH3
CH3 CH2 C CCH2 CCH3
CH3
1
2 3
4
5
6
7
6,6-Dimethyl-3-heptyne
12-9
(d)
Example 12-1, p. 333
12 Cycloalkenes
• To name a cycloalkene:
• number the carbon atoms of the ring double bond 1
and 2 in the direction that gives the lower number to
the substituent encountered first.
• number and list substituents in alphabetical order.
6
4
3
5
4
1
2
3-Meth ylcyclop entene
(not 5-methylcyclopen ten e)
© 2006 Thomson Learning, Inc.
All rights reserved
1
5
2
3
4-Ethyl-1-meth ylcyclohexen e
(not 5-ethyl-2-methylcyclohexene)
12-11
12
© 2006 Thomson Learning, Inc.
All rights reserved
12-12
Example 12-3, p. 336
12 Physical Properties
• Alkenes and alkynes are nonpolar compounds.
• The only attractive forces between their molecules are
London dispersion forces.
• Their physical properties are similar to those of
alkanes with the same carbon skeletons.
• Alkenes and alkynes are insoluble in water but soluble
in one another and in nonpolar organic liquids.
© 2006 Thomson Learning, Inc.
All rights reserved
12-13
12
Alkene Reactions
• Alkenes are quite chemically reactive
• Many reactions are addition reactions:
© 2006 Thomson Learning, Inc.
All rights reserved
12-14
12 Reactions of Alkenes
• The most common reaction is addition
D escriptive N ame(s )
Reaction
C C
C C
C C
C C
© 2006 Thomson Learning, Inc.
All rights reserved
H Cl
C C
hydrochlorin
ation
hydrohalogenation
+ H2 O
H OH
C C
hydration
+ Br
2
Br Br
C C
halogenation
bromination
+ H2
H H
C C
+
HCl
hydrogenation
(red uction)
12-15
Alkene Reactions, cont.
• Hydrogenation (addition) reactions can occur in the presence of
a catalyst (Pt, Pd, or Ni).
The hydrogenation of vegetable oils is an important
commercial process.
12 Addition of H2
• Virtually all alkenes add H2 in the presence of a
transition metal catalyst, commonly Pd, Pt, or Ni.
H3 C
H
C
+ H2
C
H
CH3
trans-2-Buten e
+ H2
Cycloh exene
© 2006 Thomson Learning, Inc.
All rights reserved
Pd
25°C, 3 atm
CH3 CH2 CH2 CH3
Butane
Pd
25°C, 3 atm
Cyclohexan e
12-17
12
Addition
of a Halogen (Halogenation)
• Addition takes place readily at room temp.
• reaction is generally carried out using pure reagents,
or mixing them in a nonreactive organic solvent
CH3 CH=CHCH3
+
Br2
2-Butene
CH2 Cl2
Br Br
CH3 CH-CHCH3
2,3-Dibromobu tan e
Br
+ Br2
Cycloh exene
CH2 Cl2
Br
1,2-Dib romocyclohexan e
• addition of Br2 is a useful qualitative test for the
presence of a carbon-carbon double bond
© 2006 Thomson Learning, Inc.
All rights reserved
12-18
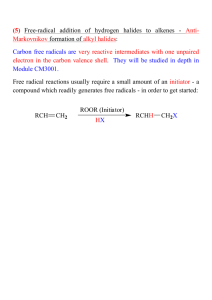
12 Addition of HX (Hydrohalogenation)
• Addition of HX (HCl, HBr, or HI) to an alkene
• H adds to one carbon of the C=C and X to the other.
CH2 =CH2
Ethylene
+
HCl
H Cl
CH2 -CH2
Chloroethane
(Ethyl chloride)
• Markovnikov’s rule: H adds to the less substituted
carbon and X to the more substituted carbon.
CH3 CH=CH2 + HCl
Prop ene
© 2006 Thomson Learning, Inc.
All rights reserved
Cl H
CH3 CH-CH2
2-Ch loroprop ane
H Cl
CH3 CH-CH2
1-Chlorop ropan e
(not formed)
12-19
12
•Hydration-addition of water
© 2006 Thomson Learning, Inc.
All rights reserved
12-20
12 Addition of H2O
• Hydration follows Markovnikov’s rule; H adds to the
less substituted carbon and OH adds to the more
substituted carbon.
CH3 CH=CH2
Propene
© 2006 Thomson Learning, Inc.
All rights reserved
+
H2 O
H2 SO4
OH H
CH3 CH-CH2
2-Propan ol
12-21
1.
4.
2.
5.
3.
Polymerization
• Polymers – long chain products made up of repeating units.
• Monomer – the starting material that becomes the repeating
units of a polymer.
Table 12-2, p. 349
12 Polymerization
• Show the structure of a polymer by placing
parentheses around the repeating monomer unit.
• Place a subscript, n, outside the parentheses to
indicate that this unit repeats n times.
• The structure of a polymer chain can be reproduced
by repeating the enclosed structure in both directions.
• following a section of polypropene (polypropylene)
© 2006 Thomson Learning, Inc.
All rights reserved
12-25