exam1key
advertisement

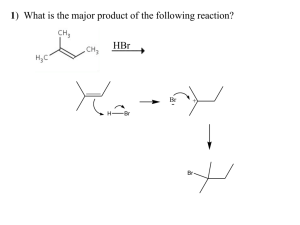
I. (29 points) Halomon (1), which was isolated from the red algae Portieria hornemannii, is a new class of antitumor agents with selective cytotoxicity against various tumor cell lines. Because of its limited availability from the natural sources, extensive efforts have been devoted toward its total synthesis for further biological studies. In a report published in 2000 by a friend of mine at Tohoku University in Japan (Angew. Chem. Int. Ed. 2000, 39, 3430), a highly efficient three-step synthesis of halomon was described. In the first step of this synthesis, monobromo-monochloro derivative 2 was prepared regioselectively from the corresponding alkene precursor in 84% yield. These polyhalogenated compounds showed intriguing spectroscopic properties. Answer the following questions concerning their NMR and mass spectroscopic behaviors. (1) (9 points) For halomon (1): Are the H’s shown below homotopic, enantiotopic, or diastereotopic to each other? (a) Two H’s at C-4: ____diastereotopic____ (b) Two H’s at C-5: ___ diastereotopic ____ (c) Two H’s at C-8: ____diastereotopic ____ (2) (8 points) For monobromo-monochloro derivative 2: 31 (a) How many different sp H chemical shifts does compound 2 have? __7___ 21 (b) How many different sp H chemical shifts does compound 2 have? ___5___ (c) How many different sp 3 13 C chemical shifts does compound 2 have? ___6___ 2 13 (d) How many different sp C chemical shifts does compound 2 have? ___4___ (3) (6 points) The mass spectrum of compound 2 (C H BrCl) shows a number of peaks corresponding to its molecular ion due to the isotopes of the two halogen atoms. Sketch below the isotope distribution of the molecular ion of 2. Show the m/Z values as well. Ignore the contributions of isotopes from non-halogen atoms. (4) (6 points) Show the mechanism for the sequential losses of the Cl and Br from the molecular ion of 2 to give the ion having the composition of C H . Each step does not need to be balanced. 10 10 16 16 Name__Key__________________________ 215H W06-Exam No. 1 Page 3 II. (6 points) In an upcoming publication (J. Am. Chem. Soc. 2006, in press), Professor Robert Grubbs of California Institute of Technology, a 2005 Nobel laureate in chemistry, describes the enantioselective synthesis of cyclic siloxane compounds, e.g., 4 from 3, with the use of chiral ruthenium olefin metathesis catalysts. 13 Indicate in the boxes provided as to how many peaks you would expect to observe in the proton-decoupled C NMR spectra of compounds 3 and 4. III. (16 points) Treatment of penta-1,4-dien-3-ol (5) with triethyl orthoacatate [CH C(OCH CH ) ] at 142 °C smoothly produces alkene 6 (C H O ) [J. Org. Chem. 2006, 71, 1085]. 3 9 14 2 3 3 2 Compound has the following spectroscopic properties: -1 IR (liquid): 1735 (strong), 1654 and 1604 cm (both medium intensity) UV (ethanol): 219 nm ( ε 18,000) [note: UV spectra of 1,3-butadiene and 1,3,5-hexatriene show λ at 214 and 244 nm, respectively)]. max 1 Η NMR (CDCl ): δ1.25 (3H, t, J = 7.1 Hz), 2.30 (2H, t, J = 7.3 Hz), 2.48 [2H, dt, J = 8.5 Hz (for d), 7.3 Hz (for t)], 4.14 (2H, q, J = 7.1 Hz), 4.99-5.75 (3 Hs, complex peak pattern), 6.09 (1H, dd, J =15.0, 3 13 10.2 Hz), 6.30 ppm (1H, ddd, J = 16.8, 10.2, 10.2 Hz). C NMR (CDCl ): δ14.1 (q), 27.7 (t), 33.7 (t), 60.2 (t), 115.0 (t), 131.8 (d), 132.5 (d), 136.7 (d), 172.7 ppm (s) [q, t, d, and s designate the peak splitting 3 13 patterns in the proton-coupled C NMR spectrum]MS: m/z 154 (molecular ion, relative intensity 43%), 109 (29%), 81 (100%), 67 (91%). (1) (2 points) What are the units of unsaturation of compound 6? ____3 (2 points) ____ 13 (2) What conclusion can be drawn from the IR absorption peak at 1735 cm and the C peak at 172.7 ppm? IV. (16 points) Certain type of dienes can undergo a Diels-Alder dimerization reaction. For example, cyclopentadiene (7) gradually produces its dimer at room temperature. (1) Provide in the box below the structure of the Diels-Alder endo product expected from cyclopentadiene (7). (2) (10 points) Draw in the boxes provided below a picture of the HOMO and LUMO (using π-type orbitals only) of cyclopendadiene (7) and explain in the boxes given briefly why a [4π+4π]-cycloaddition reaction does not take place upon heating, whereas the [4π+2π] Diels-Alder reaction proceeds. V. (30 points) Complete the following Diels-Alder cycloaddition reactions. Predict the major stereochemical and regiochemical outcomes as appropriate. Only one of an enantiomeric pair needs to be shown. V. (continued) (3) [J. Org. Chem. 2004, 69, 2084] (4) [J. Org. Chem. 2006, 71, 789] VI. (13 points) (Synthesis 2005, 2091) The reaction of diene 8 with N-phenylmaleinimide (9) produced smoothly the Diels-Alder product. Treatment of this adduct with H O /NaOH provided alcohol 10. Draw in the box provided the structure of the Diels-Alder reaction product. 2 2 (1) (2 points) Does the product you have drawn correspond to the endo-or exo-product? endo (2 points) (2) (5 points) When the borate complex 11 is used instead of diene 8, would you expect that the Diels-Alder reaction with 9 proceed faster than the one with diene 8? Provide a brief explanation in the box below with a specific comment on the HOMO energy level of 11 relative to that of 8. VII. (10 points) Heating a mixture of diene 12 and dienophile 13 results in the formation of aromatic aldehyde 14 through an intermolecular Diels-Alder reaction followed by a retro-Diels-Alder reaction. Provide in the bracket the structure of the initial Diels-Alder reaction product, including the stereochemistry. In addition, show the mechanisms of these sequential reactions using the curved-arrow convention. Draw the mechanism using the structures given in the box below.