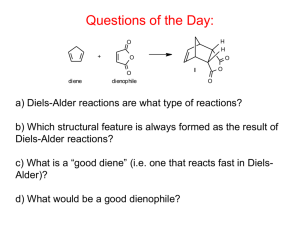
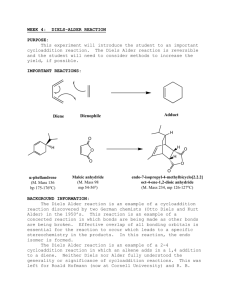
Diels-Alder. Esterification
advertisement

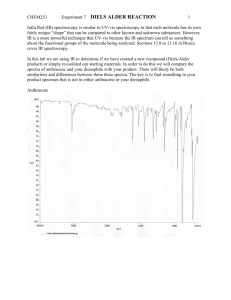
See Poster TH 7th floor hallway! Help Needed! at ACS Family Science Night at Oak Grove Middle School, Concord Wednesday, Oct.22, 6 - 9 pm What would you do? • Help set up experiments • Help middle school students (and their parents) do the experiments Please email msequin@sfsu.edu if you can help! It’s fun! What would you get? • experience in working with youngsters and showing them the fun of science (chemistry!) • a nice entry on your resume • a good impression with your instructor! Smells and Molecules Today: • Diels-Alder (cont.) H H O + diene O O O I D.-A. adduct O dienophile O • Esterification (Exp.5) a first introduction CO2 H + CH 3 OH H + CO2 CH3 + H 2 O Diels – Alder Reactions (Exp.4) Diels-Alder reactions are another example of syntheses that introduce additional C – C bonds to a reactant. Which structural feature is always formed as the result of Diels-Alder reactions? A cyclohexene ring. A stereospecific reaction, s-cis addition Diels-Alder reactions are what type of reactions? Diels – Alder Reactions The Diels-Alder reaction requires a 1,3-diene and a dienophile. 1,3-diene in s-cis conformation Dienophile: alkene, most reactive if EWG is attached Diels – Alder Reactions The Diels-Alder reaction requires a 1,3-diene (in s-cis) and a dienophile (alkene, best with EWG attached). Reaction equations for our experiment: Retro Diels Alder: Cracking of dimer H H room temp. + high temp. I Dimer dicyclopenatdiene Diels Alder: Reaction of 1,3-cyclopentadiene with maleic anhydride H H O + O O diene O Compare: Hydrolysis of Diels-Alder product H H H H2O H O O O Cis endo O I O dienophile D.-A. adduct II CO2H CO2H • Polarity of I versus II • Mp. of I versus II Diels – Alder Reactions Which of the following dienes would be most reactive? why? 1,3-cyclopentadiene, 1,3-butadiene (see problems on Report Form) Which of the following dienophiles would be more reactive? Ethene or maleic anhydride Diels – Alder Reactions Synthesis of Natural Products C A D B Steroids CH3 C CH3 A CH3 D CH3 B HO Cholesterol Diels Alder The H-NMR of the dimer of 1,3-cyclopentadiene is quite complex, with many signals. In contrast, the proton NMR of our Diels-Alder product is simple, with few signals; the same is true for its hydrolysis product. How can you explain this? emp. H H H H O O O mp. O I O dicyclopenatdiene dienophile O H H H2O H H O O I O II CO2H CO2H Methyl Benzoate Synthesis CO2 H + CH 3 OH H + CO2 CH3 + H 2 O You will heat your reaction under reflux for 1 hour! Do Practice Problems, calculations … In order to increase the yield of the ester, which of the following would help? • use an excess of methanol • use an excess of conc. sulfuric acid Calculations …! Why sulfuric acid and not HCl? Esters and Esterifications 1. General properties of esters? 2. Compare the boiling points of carboxylic acids and esters of similar molecular weight and explain. 3. Examples of esters in biological systems? • Fats and Oils • Waxes • Ripening process in fruits • phosphate esters in DNA Salicin and Aspirin OH O -D-glucose O OH C O O Salicin in willow bark (Salix) C CH 3 Next time: Conclusion of “Esterification” (Exp. 5) Grignard reactions (Exp. 6)