Tobin J. Marks Department of Chemistry and The Materials
advertisement

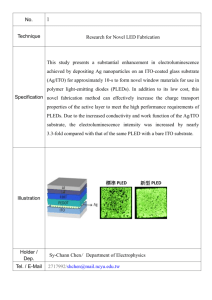
Tobin J. Marks Department of Chemistry and The Materials Research Center Northwestern University Charles E. and Emma H. Morrison Professor of Chemistry Professor of Materials Science and Engineering Vladimir N. Ipatieff Professor of Catalyic Chemistry Biographical sketch Published over 800 research articles and holds 80 U.S. patents. BS, University of Maryland, 1966 PhD, Massachusetts Institute of Technology, 1970, under Professor F. A. Cotton. Assistant Professor at Northwestern University, 1970. Numerous awards and honors. Fellow, Royal Society of Chemistry Member, National Academy of Sciences Research Activity Organometallics Photonics MOCVD Molecular Electronics Organometallics Investigate the Design and Implementation of Organometallic and Main Group Complexes for Catalysis Hydroelementation: Organo-f-element complexes for small-molecule catalysis Olefin polymerization: Nuclearity effects in group 4 and group 13 catalyst/cocatalyst studies Hydroelementation Very desirable yet challenging atom-economical transformation. Transition Metals: Impressive functional group tolerance High temperatures and catalyst decomposition Lanthanide Metals: Efficient at room temperature with long catalyst life Improving (but limited) functional group tolerance. Lanthanide Catalyzed Hydroamination Aminoalkene/alkyne hydroamination/ cyclization: Rate = k[substrate]0[Ln]1 Very sensitive to steric demands around metal center; Proposed Hydroamination Pathway Characteristics of Hydroamination Hydroamination carried out on: 1,2-disubstituted internal aminoalkene, aminoallene and aminodiene. Identical rate law for all substrates. Rate:La>Sm>Lu (aminoalkenes). Rate decreasing with increasing Ln3+ radius (aminoalkynes) and maximize at intermediate radius for aminoallenes (Y3+ >Sm3+ >Lu3+ >La3+ ). Aminoallene Cyclization Catalyst Development: Chiral Catalysts; Enantioselective Hydroamination Chiral Catalyst: C2-symmetric system Lanthanides having the largest ionic radii exhibit the greatest turnover frequencies as well as enantioselectivities. Exhibits good rates and enantioselectivities, comparable to or greater than those achieved with chiral C1symmetric organolanthanocene catalysts. Other Organolanthanide Catalyzed Hydroelementations (E = Si, B, H, P) Molecular Electronics Research involves synthesis and study of thinfilm and molecular electronic materials, focusing on the versatile thiophene-based oligomers and polymers as semiconducting and conducting layers. Utilize thin film deposition techniques like spincoating, sublimation and unique self-assembly. Addressing fundamental questions of electronic structure, optical properties and charge-transport mechanism in these materials through combined synthetic and theoretical research in collaboration with Prof. Ratner. Molecular Electronics OLED/PLED Toward the Ideal Organic LightEmitting Diode; nanoscale tailoring of the anode/HTL interfaces. OTFT Toward N-type semiconductors Gate dielectrics for lowvoltage Organic Field Effect Transistors. Organic Light Emitting Diode (I) Schematic of a typical OLED heterostructure. (II)Energy level diagram of a typical multilayer OLED. A and B indicate the cathode-ETL and anodeHTL interface, respectively. Importance of Interface Interfacial phenomena represent challenging area of OLED science. Carrier transport in OLED heterostructures largely injection limited. Focus on hole injection attenuation and electron flux enhancement. Anode-organic interface amenable to precision modification. Motivated to develop nanoscopically well defined, molecule based anode-organic (HTL) interface to remove energy discontinuity. Siloxane Based SAMs: Triarylamine Layers Characterization results from AFM, advancing aqueous contact angles, optical spectroscopy, cyclic voltammetry, XPS, UPS, and X-ray reflectivity are summarized in Table. These analyses indicate, self-limiting deposition process yields conformal, largely pinhole-free, hole-transporting molecule-scale layers of subnanometer dimensions. OLED Interfacial Structure-Charge Injection Relationships: Responses of OLEDs having structure ITO/(SAM)/NPB/AlQ:1% DIQA/2,9-dimethyl-4,7-diphenyl-1,10-phenanthroline (BCP)/Li/AgMg. (A) Current density vs voltage. (B) Luminance vs voltage. Hydrocarbon Monolayers: Contrasts with Conventional HTLs ITO/n-butylsiloxane SAM/Alq OLED exhibit luminance and efficiency comparable to TAASi3 and TPDSi2 SAM based devices. Alkyl based interlayers, thinner or thicker than C4 yield diminished performance. Triarylamine-Functionalized Anodes in Polymer Light-Emitting Diodes PLED devices: ITO/SAM or HTL/PFO/Ca/AL Triarylamine SAMs are transparent in the visible region while also offering enhanced hole injection. Fabricate blue PLED based on PFO. Maximum ext. q.e. and luminance ~ 40% and 3X greater for SAM vs PEDOT-PSS. SAM modified ITO anode enhances hole injection current 100X times vs bare ITO. Greater IP of SAM on ITO ( revealed by UPS measurements). TPD-Si2 SAM moderates discontinuity in ITO EF-PFO HOMO. Organic Field Effect Transistors The three fundamental device components are the contacts, semiconductor and the dielectric layer, typically arranged as shown in figure. Need for low voltage operating, low cost manufacture gate dielectrics. Ultrathin Cross-Linked Polymers as Gate Dielectrics for Low Voltage OFET’s Low temp fabrication, crosslinking(insolubility), ultrathin, huge k/d ratios and low leakage current. Dielectric Patterning and Capacitance. MIM and MIS leakage and capacitance measurements carried out. The current densities for CPVP-Cn and CPSCn films lower than for PVP and PS. Ultrathin CPB’s exhibit very large capacitance values. Self Assembled Multilayers (SAMTs) as Dielectric Materials Film pinhole assay by cyclic voltammetry using bare ITO (dotted line) and nanodielectrics III coated ITO as working electrodes (solid line). Measurement of nanodielectric capacitance–voltage electrical characteristics at 104 Hz. The excellent insulating properties of I–III are demonstrated by cyclic voltammetry. Towards N-type Semiconductor: Semiconducting element in organic TFT’s: high mobility, stable and solution processable. Unsubstituted,α,ω-, and β,β’- dialkyl substituted nTs and β-alkyl-substituted PTs. Carrier mobilities and on/off ratios comparable to amorphous silicon. Behave as p-type semiconductors; electron richness of thiophene. Need for N-type Semiconductor For full realization of the potential of organic electronics, high performance etransporting (n-type) materials needed. Enable applications, e.g. bipolar transistors, p-n junction diodes and complementary circuits. Varying the substitution on thiophene backbone modulates the “band gap”. Perfluoroalkyl substituted oligothiophenes First compound: DFH-6T in 2000. Intensive research towards understanding chemical, structural and physical properties as well as solidstate characteristics of five homologous series of thiophene based compounds. Optical properties Chemical substitution has minor, but core conjugation length has marked effect. Increasing number of thiophene rings and fluorocarbon substitution increase solution q.y. Lower q.y. when substituents at lateral positions. All oligothiophenes are conformationally more rigid in the e.s. Electrochemistry Electrochemistry Stability of ox and red species increase with core length and substituents at end. As core size increase ox and red parameters move to less +ve and –ve values; progressive reduction of electrochemical band gap. ΔE1/2 value decrease with increasing core length. Field Effect Transistors Field Effect Transistors All of the semiconductors investigated are FETactive, independent of the chemical substitution, regiochemistry, and core dimension. All fluorocarbonsubstituted systems functionalized at the terminal thiophene units (1 and 2) are ntype semiconductors, in contrast to the uniformly p-type activity exhibited by the remaining systems 3-5. Principal factors governing FET activity characteristics are related to the intrinsic positions of the molecular/solid-state orbitals/bands with respect to charge injection and transport. Electron-withdrawing strength of the perfluoroalkyl substituents is sufficient to lower both the fluorinated-nT HOMO and the LUMO energies such that electron injection and transport becomes, in the majority of cases, more favorable than hole injection and transport. Conclusion: Molecular Electronics OLEDs: Triarylamine based interlayers enhance HTL adhesion and afford greater hole injection fluence, higher luminance, greater external quantum efficiency and reduced turn-on voltage. Gate Dielectrics: Robust insoluble siloxane cross-linked polymeric films exhibited high capacitance and low leakage. N-type Semiconductors: Perfluorinated thiophenes were investigated and regiochemistry, core length was found to affect OFET performance. References: Organometallics: Hong, S.; Marks, T. J. Acc. Chem. Res. 2004, 37, 673-686. Molecular Electronics Veinot, J. G. C.; Marks, T. J. Acc. Chem. Res. 2004, 38, 632-643. Facchetti, A.; Yoon, M.; Marks, T. J. Adv. Mater. 2005, 17, 17051725. Facchetti, A.; Yoon, M.; Stern, C. L.; Hutchison, G. R.; Ratner, M. A.; Marks, T. J. J. Am. Chem. Soc 2004, 126, 13480-13501. Facchetti, A.; Yoon, M.; Stern, C. L.; Hutchison, G. R.; Ratner, M. A.; Marks, T. J. J. Am. Chem. Soc 2004, 126, 13859-13874.