PLOT GC Columns and Applications
The world leader in serving science
Bonded TracePLOT Columns
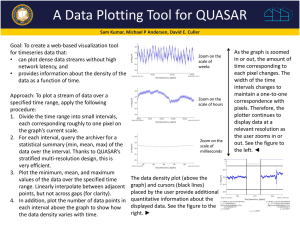
PLOT Columns - Introduction
Porous Layer Open Tubular (PLOT) GC Columns are made by coating a layer of
small particles on the inside wall of capillary tubing.
Conventional capillary columns (WCOT, Wall Coated Open Tubular) are made by
coating a layer of “liquid” phase on the inside wall of capillary tubing.
PLOT columns are the best choice for analysis of highly volatile compounds such
as permanent gases, solvents, and volatile petrochemicals such as refinery gases
PLOT phases:
Phases:
TG-BOND Alumina (Na2SO4)
TG-BOND Alumina (KCl)
TG-BOND Msieve 5A
TG-BOND Q
TG-BOND Q+
TG-BOND S
TG-BOND U
2
PLOT – Phase Polarity
Phase
Polarity
Maximum Operating Temperature
Na2SO4 Deactivated Aluminium Oxide
Non-Polar
200°C
KCl Deactivated Aluminium Oxide
Non-Polar
200°C
Molecular Sieve (5A)
Non-Polar
300°C
100% divinylbenzene
Non-Polar
280°C / 300°C
Porous divinyl benzene polymer
Mid-Polarity
250°C
Divinylbenzene 4-vinylpyridine
Mid-Polarity
250°C
Polar
190°C
TG-BOND Alumina (Na2SO4)
TG-BOND Alumina (KCl)
TR-BOND Msieve 5A
TG-BOND Q
TG-BOND Q+
TG-BOND S
Divinylbenzene ethylene glycol / dimethylacrylate
TG-BOND U
3
Increasing Polarity
TracePLOT Column
PLOT – Column Stability
“Modern” PLOT columns are engineered to provide stability and reproducibility:
Reproducibility
• Using advances in technology PLOT column manufacturers are able to accurately control the
process used to create the particles, enabling reproducible production of small particles with
uniform diameter and pore size.
Stability
• All of the particles are bonded to the tubing and/or to other particles, reducing particle generation.
This reduces or eliminates detector spiking and changes in the flow characteristics through the
column.
4
PLOT – Capillary Tubing
• Polyimide coating provides strength,
flexibility and protection from stress
corrosion caused by exposure to
moisture
• Fused silica tubing
• Control of the Fused Silica dimensions
is imperative to the performance of the
GC column
•
•
•
ID
OD
Shape
•
Surface Activity
5
PLOT – Applications
Solvent Mixture
Argon in Air
Gas Standard (H2,O2,N2,CH4,CO)
Hydrocarbons C1-C4
Hydrocarbons C1-C4
(TG-BOND Q)
(TG-BOND Msieve 5A)
(TG-BOND Msieve 5A)
(TG-BOND Alumina Na2SO4)
(TG-BOND Alumina KCl)
Application Focus:
Refinery Gas Sample
(TG-BOND Alumina Na2SO4, Alumina KCl, Q+)
6
PLOT – Solvent Mixture
800
2
24
700
1
Millivolts
600
11
6
500
21
15
4
12
23
16
400
28,29
26
20
300
13,
14
200
8
3
18
4
6
8
Column:
Part Number:
Temperature:
Detector Type:
Carrier Gas:
Flow Rate:
Injection Volume:
Injection Mode:
10
12
19
22
14
16
18
20 22
Minutes
31
25 27
30
17
5 7
100
0
9 10
24
26
28
30
TracePLOT TG-BOND Q
30m x 0.32mm x 10µm
26004-6030
100ºC to 240ºC at 5ºC/minute (10 minute hold)
FID
He
1.5 mL/min
1.0 µL
Split, 220°C
7
32
34
36
(1)
(2)
(3)
(4)
(5)
(6)
(7)
(8)
(9)
(10)
(11)
(12)
(13)
(14)
(15)
(16)
(17)
(18)
(19)
(20)
(21)
(22)
(23)
(24)
(25)
(26)
(27)
(28)
(29)
(30)
(31)
Methanol
Ethanol
Acetonitrile
Acetone
Dichloromethane
1,1,1-Trichloroethene
Nitromethane
Trans-1,2-Dichloroethene
Cis-1,2-Dichloroethene
Tetrahydrofuran
Ethyl acetate
1,2-Dichloroethane
n-Hexane
1,1,1-trichloroethane
Benzene
Trichloroethylene
1,4-Dioxane
2-Hexanone
Pyridine
N,N-Dimethylformamide
n-Heptane
Methycyclohexane
Toluene
DMSO
Chlorobenzene
N,N-Dimethylacetamide
Ethylbenzene
m-Xylene
p-Xylene
o-Xylene
Ethylene glycol 34.21min.
PLOT – Argon in Air
TRACE GC-TCD
Argon
3
120
120
100
100
80
Column:
TracePLOT TG-BOND MSieve 5A
30m x 0.53mm x 50µm
Part Number:
26003-6100
Temperature:
27ºC Isothermal
Detector Type:
TCD
Carrier Gas:
He
Flow Rate:
4.0 mL/min
Injection Volume:
1.0 µL
Injection Mode:
Split (15:1), 100°C
80
Millivolts
2
60
60
1
40
40
20
20
0
0
0.0
0.5
1.0
1.5
2.0
2.5
3.0
3.5
4.0
4.5
5.0
5.5
Minutes
1.
2.
3.
Argon
Oxygen
Nitrogen
8
6.0
PLOT – Standard Gas Mix
200
200
TRACE GC-TCD
ScottGasMix
2
Column:
TracePLOT TG-BOND MSieve 5A
30m x 0.53mm x 50µm
Part Number:
26003-6100
Temperature:
120ºC Isothermal
140
Detector Type:
TCD
120
Carrier Gas:
He
Flow Rate:
5.0 mL/min
Injection Volume:
1.0 µL
Injection Mode:
Split (12:1), 150°C
3
180
180
3
160
160
5
140
120
Millivolts
4
100
2
80
100
80
60
60
1
40
40
20
20
1
0
0
0.00
0.25
0.50
0.75
1.00
1.25
1.50
1.75
2.00
2.25
2.50
2.75
Minutes
1.
2.
3.
4.
5.
Hydrogen
Oxygen
Nitrogen
Methane
Carbon Monoxide
9
3.00
PLOT – C1-C4 Hydrocarbons
TRACE GC-FID
SplitlessPlotgas100ul_1.dat
240
6
Column:
TracePLOT TG-BOND Alumina (Na2SO4)
30m x 0.53mm x 10µm
Part Number:
26001-6080
Temperature:
40ºC (1.0 minute hold)
Ramp 1:
To 200ºC at 10ºC/minute (10 minute hold)
Detector Type:
FID
Carrier Gas:
He
Flow Rate:
40.0 mL/min
Injection Volume:
100 µL
Injection Mode:
Splitless, 180°C
220
5
200
4
Millivolts
180
160
8
7
140
2
120
100
3
1
80
60
40
0
1.
2.
3.
4.
5.
6.
7.
8.
1
2
3
4
5
6
7
8
9
Minutes
10
11
12
13
Methane
Ethane
Ethylene
Propane
Propylene
n-Butane
Acetylene
Propyne
10
14
15
PLOT – C1-C4 Hydrocarbons
1.
2.
3.
4.
5.
6.
7.
8.
Methane
Ethane
Ethylene
Propane
Propylene
n-Butane
Acetylene
Propyne
Column:
TracePLOT TG-BOND Alumina (KCl)
30m x 0.53mm x 10µm
Part Number:
26002-6080
Temperature:
40ºC (1.0 minute hold)
Ramp 1:
To 200ºC at 10ºC/minute (10 minute hold)
Detector Type:
FID
Carrier Gas:
He
Flow Rate:
40.0 mL/min
Injection Volume:
100 µL
Injection Mode:
Splitless, 180°C
Note: Change in elution order for peaks 6 & 7
11
PLOT - Refinery Gas Analysis
Porous Layer Open Tubular (PLOT) columns are well suited for the analysis of
light hydrocarbons such as those found in refinery gases. These highly
selective columns are capable of separating low molecular weight
hydrocarbons at above ambient temperatures and the columns can then be
programmed to higher temperatures to elute higher boiling compounds. The
differences in selectivity of several types of PLOT columns is demonstrated by
the differences in the separation of light hydrocarbons in a refinery gas sample.
12
PLOT - Refinery Gas Analysis-Alumina
PLOT Alumina Columns
• Alumina is often used for the analysis of volatile hydrocarbons due to its
selectivity which provides baseline resolution of most isomers at above
ambient temperatures. The highly retentive nature of alumina requires that
the surface be deactivated with inorganic salts such as sodium sulfate
(Na2SO4) or potassium chloride (KCl) to control retention.
13
PLOT - Alumina Na2SO4
F IG U R E 1 . T ra c e P L O T ™ T G -B O N D A lu m in a N a 2 S O 4 - R e fin e ry G a s
In stru m e n t – T h e rm o S cie n tific T R A C E G C
U ltra
C o lu m n : T ra ce P L O T T G -B O N D A lu m in a
N a 2 S O 4 , 3 0 m x 0 .5 3 m m ID x 2 0 u m
S a m p le – R e fin e ry G a s # 5
In je ctio n – 1 0 l (m a n u a l-syrin g e )
S p lit in je ctio n
S p lit flo w - 5 2 m L /m in (S p lit ra tio 1 3 :1 )
L in e r – 3 m m F o cu sL in e r, stra ig h t, n o g la ss
wool
In je cto r T e m p e ra tu re - 2 0 0 °C
C a rrie r G a s – H e liu m , C o n sta n t F lo w a t
4 .0 m L /m in u te
O ve n P ro g ra m - 5 0 °C (2 m in ) - 2 0 0 °C (3 m in )
a t 1 0 °C /m in
D e te ctio n – F ID 2 5 0 °C .
1.
2.
3.
4.
5.
6.
7.
M e th a n e
E th a n e
E th yle n e
P ro p a n e
P ro p yle n e
Iso b u ta n e
n -B u ta n e
8 . P ro p a d ie n e
9 . A ce tyle n e
10.
11.
12.
13.
14.
tra n s -2 -B u te n e
1 -B u te n e
Iso b u tyle n e
cis -2 -B u te n e
Iso p e n ta n e
15.
16.
17.
18.
19.
20.
n -P e n ta n e
1 ,3 -B u ta d ie n e
tra n s -2 -P e n te n e
2 -M e th yl-2 -b u te n e
1 -P e n te n e
cis -2 -P e n te n e
14
PLOT - Alumina KCl
F IG U R E 2 . T ra c e P L O T T G -B O N D A lu m in a K C l - R e fin e ry G a s
In stru m e n t – T R A C E ™ G C U ltra
C o lu m n : T ra ce P L O T T G -B O N D A lu m in a K C l,
3 0 m x 0 .5 3 m m ID x 2 0 u m
S a m p le – R e fin e ry G a s # 5
In je ctio n – 1 0 l (m a n u a l-syrin g e )
S p lit in je ctio n
S p lit flo w - 5 2 m L /m in (S p lit ra tio 1 3 :1 )
L in e r – 3 m m F o cu sL in e r, stra ig h t, n o g la ss
wool
In je cto r T e m p e ra tu re - 2 0 0 °C
C a rrie r G a s – H e liu m , C o n sta n t F lo w a t
4 .0 m L /m in u te
O ve n P ro g ra m - 5 0 °C (2 m in )-2 0 0 °C (3 m in ) a t
1 0 °C /m in
D e te ctio n – F ID 2 5 0 °C .
1.
2.
3.
4.
5.
6.
7.
M e th a n e
E th a n e
E th yle n e
P ro p a n e
P ro p yle n e
A ce tyle n e
Iso b u ta n e
8 . P ro p a d ie n e
9 . n -B u ta n e
10.
11.
12.
13.
14.
tra n s -2 -B u te n e
1 -B u te n e
Iso b u tyle n e
cis -2 -B u te n e
Iso p e n ta n e
15.
16.
17.
18.
19.
20.
n -P e n ta n e
1 ,3 -B u ta d ie n e
tra n s -2 -P e n te n e
2 -M e th yl-2 -b u te n e
1 -P e n te n e
cis -2 -P e n te n e
15
PLOT - Refinery Gas Analysis-Alumina Results
PLOT Alumina Columns
• Not shown in these chromatograms, alumina Na2SO4 elutes methyl acetylene (a.k.a.
propyne) after 1,3-butadiene, while alumina KCl elutes methyl acetylene before 1,3butadiene.
• The selectivity and retention of alumina will be affected by water, which can come
from impure carrier gas and from samples. Shorter retention times are evidence of
exposure to water. If this occurs regenerate the column by conditioning for 30
minutes at 200°C under normal carrier gas flow.
• The upper temperature limit for TracePLOT Alumina columns is 200°C. Irreversible
changes to the alumina adsorption properties will occur at higher temperatures.
16
PLOT - Refinery Gas Analysis-Porous Polymers
Porous Polymer PLOT Columns
• Porous polymer PLOT columns can also be used for the analysis of the
refinery gas sample. TracePLOT TG-BOND Q+ is a porous divinyl benzene
homopolymer of intermediate polarity incorporating a lower amount 4-vinyl
pyridine into the polymer.
• Note: Porous Polymer PLOT columns can tolerate water.
17
PLOT – Refinery Gas Analysis-Porous Polymer
F IG U R E 3 . T ra c e G O L D T G -B O N D Q + - R e fin e ry G a s
In stru m e n t – T R A C E G C U ltra
C o lu m n : T ra ce P L O T T G -B O N D Q + ,
3 0 m x 0 .5 3 m m ID x 2 0 u m
S a m p le – R e fin e ry G a s # 5
In je ctio n – 1 0 l (m a n u a l-syrin g e )
S p lit in je ctio n
S p lit flo w - 5 2 m L /m in (S p lit ra tio 1 3 :1 )
L in e r – 3 m m F o cu sL in e r, stra ig h t, n o g la ss
wool
In je cto r T e m p e ra tu re - 2 0 0 °C
C a rrie r G a s – H e liu m , C o n sta n t F lo w a t
4 .0 m L /m in u te
O ve n P ro g ra m - 5 0 °C (2 m in ) - 2 0 0 °C (3 m in )
a t 1 0 °C /m in
D e te ctio n – F ID 2 5 0 °C
1.
2.
3.
4.
5.
6.
7.
M e th a n e
E th yle n e
A ce tyle n e
E th a n e
P ro p yle n e
P ro p a n e
P ro p a d ie n e
8 . Iso b u ta n e
9 . Iso b u tyle n e
10.
11.
12.
13.
14.
1 -B u te n e
1 ,3 -B u ta d ie n e
n -B u ta n e
cis -2 -B u te n e
tra n s -2 -B u te n e
15.
16.
17.
18.
19.
20.
Iso p e n ta n e
1 -P e n te n e
tra n s -2 -P e n te n e
n -P e n ta n e
cis -2 -P e n te n e
2 -M e th yl-2 -b u te n e
18
PLOT - Conclusions
PLOT capillary columns are available in a range of phases from non-polar to polar
PLOT columns give minimal particle generation due to the particle being bonded to the
inside of the tubing. This enables greater reproducibility both run to run and column to
column.
Separation of saturated, unsaturated and branched chain hydrocarbons such as those
found in refinery gases is best accomplished on deactivated alumina PLOT columns.
Porous polymers PLOT columns are useful for analysis of volatile substances such as
solvents.
Molecular sieve PLOT columns are useful for the analysis of permanent gases.
For additional information, please visit our Chromatography Resource Centre which can
be found at: www.thermoscientific.com/chromatography
©2011 Thermo Fisher Scientific Inc. All rights reserved. All trademarks are the property of Thermo Fisher Scientific Inc. and its subsidiaries.
19